Chemistry:25-NB
The 25-NB (25x-NBx) series, or NBOMe series, also known as the N-benzylphenethylamines, is a family of serotonergic psychedelics.[1][2] They are substituted phenethylamines and were derived from the 2C family.[2] The most commonly encountered NBOMe drugs are 25I-NBOMe, 25B-NBOMe, and 25C-NBOMe.[3]
The NBOMe drugs act as selective agonists of the serotonin 5-HT2 receptors.[4][5][6][7][8][9][10] The 25-NB family is unique relative to other classes of psychedelics in that they are, generally speaking, extremely potent and quite selective for the 5-HT2 receptors.[2]
Use of NBOMe series drugs has caused many deaths and hospitalisations since the drugs popularisation in the 2010s. This is primarily due to their high potency, unpredictable pharmacokinetics, and sellers passing off the compounds in the series as LSD.[11]
Use and effects
The 25-NB drugs are inactive orally and instead are typically used sublingually, buccally, by insufflation, or sometimes via inhalation.[12][3][13] They are typically employed at doses in the range of 50 to 1,500 μg, variable depending on the specific drug, and have durations in the range of 3 to 12 hours.[3][13][12][14][15] The table below provides an overview of the major 25-NB drugs and their properties.[3][13][12][14][15]
| Compound | Chemical name | Dose | Duration |
|---|---|---|---|
| 25B-NBOMe | N-(2-Methoxybenzyl)-4-bromo-2,5-dimethoxyphenethylamine | 50–700+ μg | 8–12 hours |
| 25C-NBOMe | N-(2-Methoxybenzyl)-4-chloro-2,5-dimethoxyphenethylamine | 50–1,250+ μg | 3–10 hours |
| 25D-NBOMe | N-(2-Methoxybenzyl)-4-methyl-2,5-dimethoxyphenethylamine | 300–1,200+ μg | Unknown |
| 25E-NBOMe | N-(2-Methoxybenzyl)-4-ethyl-2,5-dimethoxyphenethylamine | 50–800 μg | 5–10 hours |
| 25H-NBOMe | N-(2-Methoxybenzyl)-2,5-dimethoxyphenethylamine | Unknown | Unknown |
| 25I-NBOMe | N-(2-Methoxybenzyl)-4-iodo-2,5-dimethoxyphenethylamine | 50–1,200 μg | 4–10 hours |
| 25N-NBOMe | N-(2-Methoxybenzyl)-4-nitro-2,5-dimethoxyphenethylamine | 100–1,300+ μg | 5–10 hours |
| 25P-NBOMe | N-(2-Methoxybenzyl)-4-propyl-2,5-dimethoxyphenethylamine | 100–1,500 μg | Unknown |
| 25T2-NBOMe | N-(2-Methoxybenzyl)-4-ethylthio-2,5-dimethoxyphenethylamine | 100–1,000 μg | Unknown |
| 25T4-NBOMe | N-(2-Methoxybenzyl)-4-isopropylthio-2,5-dimethoxyphenethylamine | 150–1,200 μg | Unknown |
| 25T7-NBOMe | N-(2-Methoxybenzyl)-4-propylthio-2,5-dimethoxyphenethylamine | Unknown | Unknown |
| Notes: The route of administration is sublingual, insufflation, and/or buccal.[3][13][12] They may also be smoked.[12] NBOMe drugs have very low oral bioavailability.[12] Refs: [3][13][12][16][17][18][14][15][19] | |||
Toxicity and harm potential
NBOMe compounds are often associated with life-threatening toxicity and death.[20][21] Studies on NBOMe family of compounds demonstrated that the substance exhibit neurotoxic and cardiotoxic activity.[3] Reports of autonomic dysfunction remains prevalent with NBOMe compounds, with most individuals experiencing sympathomimetic toxicity such as vasoconstriction, hypertension and tachycardia in addition to hallucinations.[22][13][23][24][25] Other symptoms of toxidrome include agitation or aggression, seizure, hyperthermia, diaphoresis, hypertonia, rhabdomyolysis, and death.[22][25][21] Researchers report that NBOMe intoxication frequently display signs of serotonin syndrome.[26] The likelihood of seizure is higher in NBOMes compared to other psychedelics.[3]
NBOMe and NBOHs are regularly sold as LSD in blotter papers,[21][27] which have a bitter taste and different safety profiles.[22][20] Despite high potency, recreational doses of LSD have only produced low incidents of acute toxicity.[20] Fatalities involved in NBOMe intoxication suggest that a significant number of individuals ingested the substance which they believed was LSD,[23] and researchers report that "users familiar with LSD may have a false sense of security when ingesting NBOMe inadvertently".[22] While most fatalities are due to the physical effects of the drug, there have also been reports of death due to self-harm and suicide under the influence of the substance.[28][29][22]
Given limited documentation of NBOMe consumption, the long-term effects of the substance remain unknown.[22] NBOMe compounds are not active orally,[lower-alpha 1] and are usually taken sublingually.[2]: 3 When NBOMes are administered sublingually, numbness of the tongue and mouth followed by a metallic chemical taste was observed, and researchers describe this physical side effect as one of the main discriminants between NBOMe compounds and LSD.[31][15][32]
Neurotoxic and cardiotoxic actions
Many of the NBOMe compounds have high potency agonist activity at additional 5-HT receptors and prolonged activation of 5-HT2B can cause cardiac valvulopathy in high doses and chronic use.[21][24] 5-HT2B receptors have been strongly implicated in causing drug-induced valvular heart disease.[33][34][35] The high affinity of NBOMe compounds for adrenergic α1 receptor has been reported to contribute to the stimulant-type cardiovascular effects.[24]
In vitro studies, 25C-NBOMe has been shown to exhibit cytotoxicity on neuronal cell lines SH-SY5Y, PC12, and SN471, and the compound was more potent than methamphetamine at reducing the visibility of the respective cells; the neurotoxicity of the compound involves activation of MAPK/ERK cascade and inhibition of Akt/PKB signaling pathway.[3] 25C-NBOMe, including the other derivative 25D-NBOMe, reduced the visibility of cardiomyocytes H9c2 cells, and both substances downregulated expression level of p21 (CDC24/RAC)-activated kinase 1 (PAK1), an enzyme with documented cardiac protective effects.[3]
Preliminary studies on 25C-NBOMe have shown that the substance is toxic to development, heart health, and brain health in zebrafish, rats, and Artemia salina, a common organism for studying potential drug effects on humans, but more research is needed on the topic, the dosages, and if the toxicology results apply to humans. Researchers of the study also recommended further investigation of the drug's potential in damaging pregnant women and their fetus due to the substance's damaging effects to development.[36][37]
Emergency treatment
At present, there are no specific antidotes for NBOMes, and all acute intoxication is managed by symptomatic treatments, such as administration of benzodiazepines, antipsychotic drugs, and antiarrhythmic agents, such as beta blockers; some emergency interventions are intended to specifically treat rhabdomyolysis, which may lead to critical complications such as metabolic acidosis and acute kidney injury.[3]
Interactions
2C drugs like 2C-I are metabolized by the monoamine oxidase (MAO) enzymes, including both MAO-A and MAO-B.[38][39] As a result, 2C drugs may be potentiated by monoamine oxidase inhibitors (MAOIs), such as phenelzine, tranylcypromine, moclobemide, and selegiline.[38][39][40] This has the potential to lead to overdose and serious toxicity.[38][39][40] In contrast to 2C drugs, 25I-NBOMe has been found not to be metabolized by MAO-A or MAO-B and instead only by cytochrome P450 enzymes.[41] Other 25-NB drugs besides 25I-NBOMe were not assessed.[41]
Pharmacology
Pharmacodynamics
Actions
The NBOMe drugs are highly potent and selective agonists of the serotonin 5-HT2 receptors, including of the 5-HT2A, 5-HT2B, and 5-HT2C receptors.[1][3][42][43][44] However, they are much less potent and efficacious at the serotonin 5-HT2B receptor compared to the serotonin 5-HT2A and 5-HT2C receptors.[3][44] The drugs are highly selective for the serotonin 5-HT2 receptors over other serotonin receptors and over a variety of other biological targets.[1][3][42][43] They are likewise inactive as monoamine reuptake inhibitors and releasing agents.[42] Many of the NBOMe drugs are partial agonists of the rat and mouse trace amine-associated receptor 1 (TAAR1), but they are inactive as agonists of the human TAAR1.[45]
Effects
In accordance with their psychedelic effects, NBOMe drugs induce the head-twitch response, a behavioral proxy of psychedelic effects, in rodents.[46] They have also been found to produce hyperlocomotion at low doses and hypolocomotion at high doses in rodents.[46]
Unlike most other serotonergic psychedelics, the NBOMe drugs 25B-NBOMe and 25N-NBOMe have been found to produce reinforcing effects in rodents, and hence may have misuse potential.[3][47][48] Relatedly, 25B-NBOMe robustly increased dopamine levels in the nucleus accumbens similarly to methamphetamine.[3][47] The reinforcing effects of 25B-NBOMe were not blocked by serotonin 5-HT2A receptor antagonism, and it is unclear how they are produced.[3][47] However, some NBOMe drugs, such as 25N-NBOMe, have been found to increase phosphorylation of the dopamine transporter (DAT) in the striatum similarly to methamphetamine in rodents.[49][48] DAT phosphorylation is associated with dopamine reverse transport and efflux, which in turn increases extracellular dopamine levels.[49][48]
Similarly to other psychedelics like DOI and 2C-T-7, tolerance has been found to gradually develop to the head-twitch response induced by 25I-NBOMe with chronic administration in rodents.[46][50]
No human clinical data exist on the pharmacology of NBOMe derivatives as of 2020.[51]
Chemistry
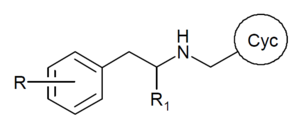
The 25-NB compounds are mostly N-benzylphenethylamines,[2][1] though in some cases the phenyl ring of the N-benzyl group is replaced by other heterocycles such as thiophene, pyridine, furan, tetrahydrofuran, benzodioxole or naphthalene, among others.[52][53]
Generally speaking, they have methoxy groups at the 2 and 5 positions of the phenyl ring, a substitution such as a halogen or alkyl group at the 4 position of the phenyl ring, and a methoxy or other substitution (e.g., hydroxyl, fluoro) at the 2 position of the N-benzyl ring.[2] More rarely, other substitution patterns may be present [54][55] (see e.g. NBOMe-mescaline, 25G-NBOMe, 2CBFly-NBOMe, 25C-NB3OMe). They differ from the 2C series by the presence of the N-benzyl moiety.[2]
Rarely an alpha-methyl group is present making them N-benzyl amphetamines rather than N-benzyl phenethylamines, but this greatly reduces potency and activity. However in some cases where a side chain methyl group is cyclised back to the ring (e.g. in 2CBCB-NBOMe) or links the two alpha positions (e.g. in DMBMPP), this can improve selectivity for the 5-HT2A receptor subtype.[56]
History
2C-B, the first major 2C drug and an analogue of mescaline, was first described by Alexander Shulgin in the 1970s.[1] Richard Glennon and colleagues synthesized and described 25B-NB (N-benzyl-2C-B) along with a variety of other 25-NB derivatives in 1994.[57][1][58] It was observed at the time that 25B-NB had slightly higher affinity for the serotonin 5-HT2A receptor than 2C-B and that other 25-NB derivatives with substituents on the benzyl ring showed very high affinity for the receptor, though functional data were not reported.[57][58]
N-Benzyl derivatives of the ketanserin-related quinazolinedione EZS-8, such as RH-34, were first described by Heinz Pertz, Sigurd Elz, and Ralf Heim by 1996 or 1998.[59][60][61] NBOMe-mescaline and NBOMe-escaline were first described by Pertz and colleagues by 1999,[62][63] while 25B-NBOMe was first described by Heim and colleagues in 1999.[64][65] 25I-NBOMe and other 25-NB compounds such as 25TFM-NBOMe and 2CBFly-NBOMe were described by Heim and colleagues by 2000.[66][67][68][69] 25I-NBOMe and other 25-NB drugs were subsequently further described by Heim in his dissertation in 2003.[69] 25C-NBOMe was not described in the literature until 2010.[70][71] The discovery of the 25-NB compounds by Heim and colleagues has been described by David E. Nichols as structurally remarkable, since N-alkylation of psychedelic phenethylamines, for instance Beatrice (N-methyl-DOM), has otherwise invariably abolished the hallucinogenic effects of this class of compounds.[64]
The NBOMe drugs, primarily 25I-NBOMe, were encountered as novel recreational drugs by 2010, and by 2012 had eclipsed other psychedelics like LSD and psilocybin-containing mushrooms in popularity, at least for a time.[1][72][73][74] Various NBOMes, such as 25I-NBOMe, became Schedule I controlled substances in the United States in 2013.[1]
Society and culture
Legal status
United Kingdom
A large number of substances in the 25-NB class are Class A drugs in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971[75] or are otherwise covered by the Psychoactive Substances Act 2016.[76]
List of 25-NB compounds
By chemical class
| Group | Compounds |
|---|---|
| 25x-NB | 25B-NB • 25C-NB • 25I-NB |
| 25x-NB3OMe | 25B-NB3OMe • 25C-NB3OMe • 25D-NB3OMe • 25E-NB3OMe • 25H-NB3OMe • 25I-NB3OMe • 25N-NB3OMe • 25P-NB3OMe • 25T2-NB3OMe • 25T4-NB3OMe • 25T7-NB3OMe • 25TFM-NB3OMe |
| 25x-NB4OMe | 25B-NB4OMe • 25C-NB4OMe • 25D-NB4OMe • 25E-NB4OMe • 25H-NB4OMe • 25I-NB4OMe • 25N-NB4OMe • 25P-NB4OMe • 25T2-NB4OMe • 25T4-NB4OMe • 25T7-NB4OMe • 25TFM-NB4OMe |
| 25x-NBF | 25B-NBF • 25C-NBF • 25D-NBF • 25E-NBF • 25H-NBF • 25I-NBF • 25P-NBF • 25T2-NBF • 25T7-NBF • 25TFM-NBF |
| 25x-NBOH | 25B-NBOH • 25C-NBOH • 25CN-NBOH • 25D-NBOH • 25E-NBOH • 25F-NBOH • 25H-NBOH • 25I-NBOH • 25P-NBOH • 25T2-NBOH • 25T7-NBOH • 25TFM-NBOH |
| 25x-NBOMe | 25B-NBOMe • 25C-NBOMe • 25CN-NBOMe • 25D-NBOMe • 25E-NBOMe • 25F-NBOMe • 25G-NBOMe • 25H-NBOMe • 25I-NBOMe • 25iP-NBOMe • 25N-NBOMe • 25O-NBOMe • 25P-NBOMe • 25T-NBOMe • 25T2-NBOMe • 25T4-NBOMe • 25T7-NBOMe • 25TFM-NBOMe |
| 25x-NBMD | 25B-NBMD • 25C-NBMD • 25D-NBMD • 25E-NBMD • 25F-NBMD • 25H-NBMD • 25I-NBMD • 25P-NBMD • 25T2-NBMD • 25T7-NBMD • 25TFM-NBMD |
| Atypical 25-NB structures | 25B-N1POMe • 25B-NAcPip • 25B-NB23DM • 25B-NB25DM • 25C-NBCl • 25C-NBOEt • 25C-NBOiPr • 25I-N2Nap1OH • 25I-N3MT2M • 25I-N4MT3M • 25I-NB34MD • 25I-NBAm • 25I-NBBr • 25I-NBMeOH • 25I-NBTFM • 2C2-NBOMe • 2CBCB-NBOMe • 2CBFly-NBOMe • 4-EA-NBOMe • 5-APB-NBOMe • 5MT-NBOMe • 5MT-NB3OMe • C30-NBOMe • DOB-NBOMe • DOI-NBOMe • DOM-NBOMe • FECIMBI-36 • MDPEA-NBOMe • NBOMe-escaline • NBOMe-mescaline • ZDCM-04 |
By individual compound
This list includes notable compounds representative of most of the structural variations that have been explored in this series, but is by no means exhaustive. Many derivatives invented for scientific study into the structure-activity relationships of 5-HT2 receptor agonists have never appeared as designer drugs, while conversely some derivatives that have appeared as designer drugs are structurally novel and of unknown pharmacological activity (e.g. C30-NBOMe, 5-APB-NBOMe).
| Structure | Name | Chemical name | CAS # | R | R1 | Cyc |
|---|---|---|---|---|---|---|
|
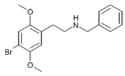
25B-NB | N-benzyl-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 155639-26-2 | 2,5-dimethoxy-4-bromo | H | phenyl |

|
25C-NB | N-benzyl-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1391487-65-2 | 2,5-dimethoxy-4-chloro | H | phenyl |

|
25I-NB | N-benzyl-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-18-5 | 2,5-dimethoxy-4-iodo | H | phenyl |
|
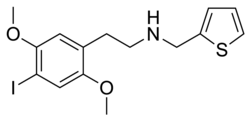
25I-NMeTh | N-[(thiophen-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391499-03-8 | 2,5-dimethoxy-4-iodo | H | thiophen-2-yl |
|
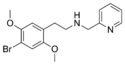
25B-NMePyr | N-[(pyridin-2-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391499-21-0 | 2,5-dimethoxy-4-bromo | H | pyridin-2-yl |
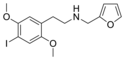
|
25I-NMeFur | N-[(furan-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391498-93-3 | 2,5-dimethoxy-4-iodo | H | furan-2-yl |
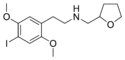
|
25I-NMeTHF | N-[(tetrahydrofuran-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | tetrahydrofuran-2-yl | |
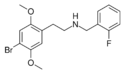
|
25B-NBF | N-(2-fluorobenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1539266-17-5 | 2,5-dimethoxy-4-bromo | H | 2-fluorophenyl |
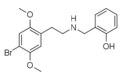
|
25B-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1335331-46-8 | 2,5-dimethoxy-4-bromo | H | 2-hydroxyphenyl |
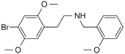
|
25B-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1026511-90-9 | 2,5-dimethoxy-4-bromo | H | 2-methoxyphenyl |

|
25B-NB23DM | N-(2,3-dimethoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391493-68-7 | 2,5-dimethoxy-4-bromo | H | 2,3-dimethoxyphenyl |
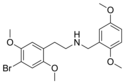
|
25B-NB25DM | N-(2,5-dimethoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 2,5-dimethoxy-4-bromo | H | 2,5-dimethoxyphenyl | |
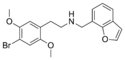
|
25B-NMe7BF | N-[(benzofuran-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391492-46-8 | 2,5-dimethoxy-4-bromo | H | benzofuran-7-yl |
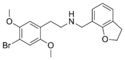
|
25B-NMe7DHBF | N-[(2,3-dihydrobenzofuran-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391492-40-2 | 2,5-dimethoxy-4-bromo | H | 2,3-dihydrobenzofuran-7-yl |
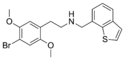
|
25B-NMe7BT | N-[(benzothiophen-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391492-59-3 | 2,5-dimethoxy-4-bromo | H | benzothiophen-7-yl |
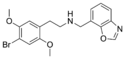
|
25B-NMe7Box | N-[(benzoxazol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-73-9 | 2,5-dimethoxy-4-bromo | H | benzoxazol-7-yl |
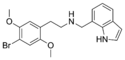
|
25B-NMe7Ind | N-[(indol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-28-4 | 2,5-dimethoxy-4-bromo | H | indol-7-yl |
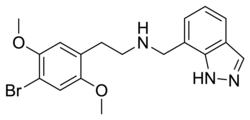
|
25B-NMe7Indz | N-[(indazol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-43-3 | 2,5-dimethoxy-4-bromo | H | indazol-7-yl |
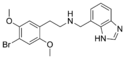
|
25B-NMe7Bim | N-[(benzimidazol-7-yl)methyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1391498-62-6 | 2,5-dimethoxy-4-bromo | H | benzimidazol-7-yl |
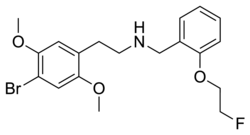
|
FECIMBI-36 | N-[(2-fluoroethoxy)benzyl]-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 2,5-dimethoxy-4-bromo | H | 2-(2-fluoroethoxy)phenyl | |
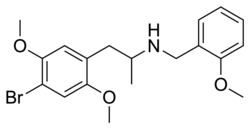
|
DOB-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane | 2,5-dimethoxy-4-bromo | methyl | 2-methoxyphenyl | |
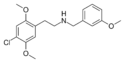
|
25C-NB3OMe | N-(3-methoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1566571-34-3 | 2,5-dimethoxy-4-chloro | H | 3-methoxyphenyl |
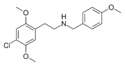
|
25C-NB4OMe | N-(4-methoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1566571-35-4 | 2,5-dimethoxy-4-chloro | H | 4-methoxyphenyl |
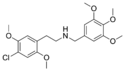
|
C30-NBOMe | N-(3,4,5-trimethoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1445574-98-0 | 2,5-dimethoxy-4-chloro | H | 3,4,5-trimethoxyphenyl |
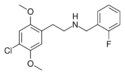
|
25C-NBF | N-(2-fluorobenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1539266-21-1 | 2,5-dimethoxy-4-chloro | H | 2-fluorophenyl |
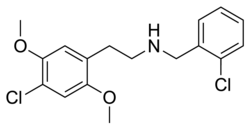
|
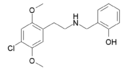
25C-NBCl | N-(2-chlorobenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 2,5-dimethoxy-4-chloro | H | 2-chlorophenyl | |
|
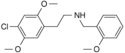
25C-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1391488-16-6 | 2,5-dimethoxy-4-chloro | H | 2-hydroxyphenyl |
|
25C-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1227608-02-7 | 2,5-dimethoxy-4-chloro | H | 2-methoxyphenyl |

|
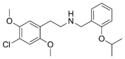
25C-NBOEt | N-(2-ethoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 2,5-dimethoxy-4-chloro | H | 2-ethoxyphenyl | |
|
25C-NBOiPr | N-(2-isopropoxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 2,5-dimethoxy-4-chloro | H | 2-isopropoxyphenyl | |

|
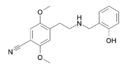
25F-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-fluorophenyl)-2-aminoethane | 1373917-84-0 | 2,5-dimethoxy-4-fluoro | H | 2-methoxyphenyl |
|
25CN-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-cyanophenyl)-2-aminoethane | 1539266-32-4 | 2,5-dimethoxy-4-cyano | H | 2-hydroxyphenyl |
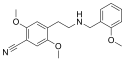
|
25CN-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-cyanophenyl)-2-aminoethane | 1354632-16-8 | 2,5-dimethoxy-4-cyano | H | 2-methoxyphenyl |
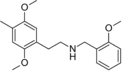
|
25D-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminoethane | 1354632-02-2 | 2,5-dimethoxy-4-methyl | H | 2-methoxyphenyl |
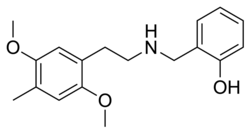
|
25D-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminoethane | 1391488-44-0 | 2,5-dimethoxy-4-methyl | H | 2-hydroxyphenyl |
| Error creating thumbnail: | DOM-NBOMe | N-(2-methoxybenzyl)-4-methyl-2,5-dimethoxy-2-aminopropane | 2836395-73-2 | 2,5-dimethoxy-4-methyl | methyl | 2-methoxyphenyl |
|
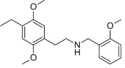
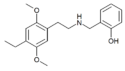
25E-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-ethylphenyl)-2-aminoethane | 1354632-14-6 | 2,5-dimethoxy-4-ethyl | H | 2-methoxyphenyl |
|
25E-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-ethylphenyl)-2-aminoethane | 1391489-79-4 | 2,5-dimethoxy-4-ethyl | H | 2-hydroxyphenyl |
|
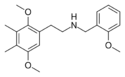
25G-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-3,4-dimethylphenyl)-2-aminoethane | 1354632-65-7 | 2,5-dimethoxy-3,4-dimethyl | H | 2-methoxyphenyl |
|
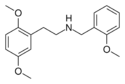
25H-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxyphenyl)-2-aminoethane | 1566571-52-5 | 2,5-dimethoxy | H | 2-methoxyphenyl |
|
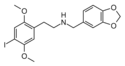
25I-NB34MD | N-(3,4-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391497-81-6 | 2,5-dimethoxy-4-iodo | H | 3,4-methylenedioxyphenyl |
|
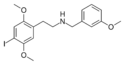
25I-NB3OMe | N-(3-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1566571-40-1 | 2,5-dimethoxy-4-iodo | H | 3-methoxyphenyl |
|
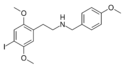
25I-NB4OMe | N-(4-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1566571-41-2 | 2,5-dimethoxy-4-iodo | H | 4-methoxyphenyl |
|
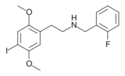
25I-NBF | N-(2-fluorobenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-21-0 | 2,5-dimethoxy-4-iodo | H | 2-fluorophenyl |
|
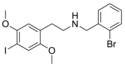
25I-NBBr | N-(2-bromobenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1648649-98-2 | 2,5-dimethoxy-4-iodo | H | 2-bromophenyl |
| Error creating thumbnail: | 25I-NBTFM | N-[2-(trifluoromethyl)benzyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | 2-(trifluoromethyl)phenyl | |
|
25I-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-25-4 | 2,5-dimethoxy-4-iodo | H | 2,3-methylenedioxyphenyl |
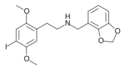
| Error creating thumbnail: | 25B-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminoethane | 1354632-19-1 | 2,5-dimethoxy-4-bromo | H | 2,3-methylenedioxyphenyl |
|
25C-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-chlorophenyl)-2-aminoethane | 1373879-26-5 | 2,5-dimethoxy-4-chloro | H | 2,3-methylenedioxyphenyl |
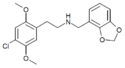
| Error creating thumbnail: | 25D-NBMD | N-(2,3-methylenedioxybenzyl)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminoethane | 1391488-97-3 | 2,5-dimethoxy-4-methyl | H | 2,3-methylenedioxyphenyl |
|
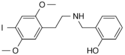
25I-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-20-9 | 2,5-dimethoxy-4-iodo | H | 2-hydroxyphenyl |
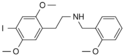
|
25I-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 919797-19-6 | 2,5-dimethoxy-4-iodo | H | 2-methoxyphenyl |
| Error creating thumbnail: | DOI-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane | 2,5-dimethoxy-4-iodo | methyl | 2-methoxyphenyl | |
| Error creating thumbnail: | 25I-NBMeOH | N-[2-(hydroxymethyl)benzyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391494-71-5 | 2,5-dimethoxy-4-iodo | H | 2-(hydroxymethyl)phenyl |
| Error creating thumbnail: | 25I-NBAm | N-[2-(carbamoyl)benzyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1391494-85-1 | 2,5-dimethoxy-4-iodo | H | 2-(carbamoyl)phenyl |
| Error creating thumbnail: | 25I-NMe7DHBF | N-[(2,3-dihydrobenzofuran-7-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | 2,3-dihydrobenzofuran-7-yl | |
| Error creating thumbnail: | 25I-N2Nap1OH | N-[(1-hydroxynaphthalen-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 2,5-dimethoxy-4-iodo | H | 1-hydroxynaphthalen-2-yl | |
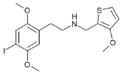
|
25I-N3MT2M | N-[(3-methoxythiophen-2-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1354632-66-8 | 2,5-dimethoxy-4-iodo | H | 3-methoxythiophen-2-yl |
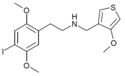
|
25I-N4MT3M | N-[(4-methoxythiophen-3-yl)methyl]-1-(2,5-dimethoxy-4-iodophenyl)-2-aminoethane | 1354632-73-7 | 2,5-dimethoxy-4-iodo | H | 4-methoxythiophen-3-yl |
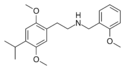
|
25iP-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-isopropylphenyl)-2-aminoethane | 1391487-83-4 | 2,5-dimethoxy-4-isopropyl | H | 2-methoxyphenyl |
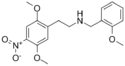
|
25N-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 1354632-03-3 | 2,5-dimethoxy-4-nitro | H | 2-methoxyphenyl |
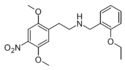
|
25N-NBOEt [77] | N-(2-ethoxybenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 2-ethoxyphenyl | |
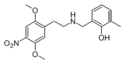
|
25N-NB-2-OH-3-Me | N-(2-hydroxy-3-methylbenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 2-hydroxy-3-methylphenyl | |
| Error creating thumbnail: | 25N-NBOCF2H | N-(2-difluoromethoxybenzyl)-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 2-difluoromethoxyphenyl | |
| Error creating thumbnail: | 25N-NBPh[78] | N-[(2-phenyl)benzyl]-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | o-biphenyl | |
| Error creating thumbnail: | 25N-N1-Nap | N-[(naphthalen-1-yl)methyl]-1-(2,5-dimethoxy-4-nitrophenyl)-2-aminoethane | 2,5-dimethoxy-4-nitro | H | 1-naphthyl | |
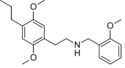
|
25P-NBOMe | N-(2-methoxybenzyl)-1-(2,5-dimethoxy-4-propylphenyl)-2-aminoethane | 1391489-07-8 | 2,5-dimethoxy-4-propyl | H | 2-methoxyphenyl |
| Error creating thumbnail: | 25P-NBOH | N-(2-hydroxybenzyl)-1-(2,5-dimethoxy-4-propylphenyl)-2-aminoethane | 1391490-34-8 | 2,5-dimethoxy-4-propyl | H | 2-hydroxyphenyl |
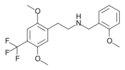
|
25TFM-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(trifluoromethyl)phenyl]-2-aminoethane | 1027161-33-6 | 2,5-dimethoxy-4-(trifluoromethyl) | H | 2-methoxyphenyl |
| Error creating thumbnail: | 25O-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-methoxyphenyl]-2-aminoethane | ? | 2,5-dimethoxy-4-methoxy | H | 2-methoxyphenyl |
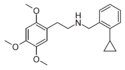
|
25O-NBcP | N-(2-cyclopropylbenzyl)-1-(2,4,5-trimethoxyphenyl)-2-aminoethane | 2,4,5-trimethoxy | H | 2-cyclopropylphenyl | |

|
RS130-180 | N-(2-propargyloxy)-2,5-dimethoxy-4-(dimethylamino)phenethylamine | 4-(N,N-dimethylamino) | H | 2-(prop-2-yn-1-yloxy)phenyl | |

|
25T-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(methylthio)phenyl]-2-aminoethane | 1539266-47-1 | 2,5-dimethoxy-4-(methylthio) | H | 2-methoxyphenyl |
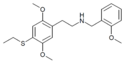
|
25T2-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(ethylthio)phenyl]-2-aminoethane | 1539266-51-7 | 2,5-dimethoxy-4-(ethylthio) | H | 2-methoxyphenyl |
| Error creating thumbnail: | 25T4-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(isopropylthio)phenyl]-2-aminoethane | 1354632-17-9 | 2,5-dimethoxy-4-(isopropylthio) | H | 2-methoxyphenyl |
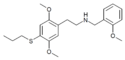
|
25T7-NBOMe | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-(propylthio)phenyl]-2-aminoethane | 1539266-55-1 | 2,5-dimethoxy-4-(propylthio) | H | 2-methoxyphenyl |
| Error creating thumbnail: | 25T7-NBOH | N-(2-hydroxybenzyl)-1-[2,5-dimethoxy-4-(propylthio)phenyl]-2-aminoethane | 1354632-41-9 | 2,5-dimethoxy-4-(propylthio) | H | 2-hydroxyphenyl |

|
25T-TFM-NCPM [79] | N-cyclopropylmethyl-1-[2,5-dimethoxy-4-(trifluoromethylthio)phenyl]-2-aminoethane | 2,5-dimethoxy-4-(trifluoromethylthio) | H | cyclopropylmethyl | |
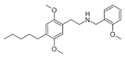
|
25AM-NBOMe [80] | N-(2-methoxybenzyl)-1-[2,5-dimethoxy-4-pentylphenyl]-2-aminoethane | 2,5-dimethoxy-4-(n-pentyl) | H | 2-methoxyphenyl | |
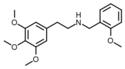
|
NBOMe-mescaline | N-(2-methoxybenzyl)-1-(3,4,5-trimethoxyphenyl)-2-aminoethane | 1354632-01-1 | 3,4,5-trimethoxy | H | 2-methoxyphenyl |
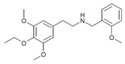
|
NBOMe-escaline | N-(2-methoxybenzyl)-1-(3,5-dimethoxy-4-ethoxyphenyl)-2-aminoethane | 3,5-dimethoxy-4-ethoxy | H | 2-methoxyphenyl | |
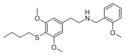
|
NBOMe-thiobuscaline | N-(2-methoxybenzyl)-1-(3,5-dimethoxy-4-butylthiophenyl)-2-aminoethane | 3,5-dimethoxy-4-(n-butylthio) | H | 2-methoxyphenyl | |
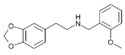
|
MDPEA-NBOMe | N-(2-methoxybenzyl)-1-(3,4-methylenedioxyphenyl)-2-aminoethane | 3,4-methylenedioxy | H | 2-methoxyphenyl | |

|
Lophophine-NBOMe (MMDPEA-NBOMe) | N-(2-methoxybenzyl)-1-(3-methoxy-4,5-methylenedioxyphenyl)-2-aminoethane | 3-methoxy-4,5-methylenedioxy | H | 2-methoxyphenyl | |
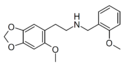
|
2C2-NBOMe (MMDPEA-2-NBOMe) | N-(2-methoxybenzyl)-1-(2-methoxy-4,5-methylenedioxyphenyl)-2-aminoethane | 2-methoxy-4,5-methylenedioxy | H | 2-methoxyphenyl | |
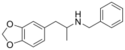
|
MDBZ | N-benzyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane | 65033-29-6 | 3,4-methylenedioxy | methyl | phenyl |
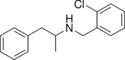
|
Clobenzorex | N-(2-chlorobenzyl)-1-phenyl-2-aminopropane | 13364-32-4 | H | methyl | 2-chlorophenyl |
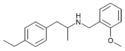
|
4-EA-NBOMe | N-(2-methoxybenzyl)-1-(4-ethylphenyl)-2-aminopropane | 4-ethyl | methyl | 2-methoxyphenyl | |
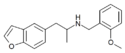
|
5-APB-NBOMe | N-(2-methoxybenzyl)-1-(benzofuran-5-yl)-2-aminopropane | benzofuran-5-yl instead of phenyl | methyl | 2-methoxyphenyl |
Related compounds
Similar compounds with related structures are also known including:
| Structure | Name | Chemical name | CAS # |
|---|---|---|---|
| Error creating thumbnail: | 25B-N1POMe | N-[1-(2-methoxyphenyl)ethyl]-2,5-dimethoxy-4-bromophenethylamine | 1335331-49-1 (R) 1335331-51-5 (S) |

|
TGF-8027 | N-[1-(2-hydroxyphenyl)ethyl]-2,5-dimethoxy-4-cyanophenethylamine | ? |
| Error creating thumbnail: | 2C-B-AN [81][82] | 2-phenyl-2-[2-(2,5-dimethoxy-4-bromophenyl)ethylamino]acetonitrile | |
| Error creating thumbnail: | 25B-N(BOMe)2[83] | 2-(4-Bromo-2,5-dimethoxyphenyl)-N,N-bis(2-methoxybenzyl)ethan-1-amine | |

|
25CN-N3DHBF[84] | 4-(2-[(2,3-dihydro-1-benzofuran-3-yl)amino]ethyl)-2,5-dimethoxybenzonitrile | |
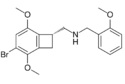
|
2CBCB-NBOMe | N-[(3-bromo-2,5-dimethoxy-bicyclo[4,2,0]octa-1,3,5-trien-7-yl)methyl]-1-(2-methoxyphenyl)methanamine | 1354634-09-5 |
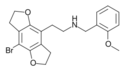
|
2CBFly-NBOMe | N-(2-methoxybenzyl)-1-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane | 1335331-42-4 |
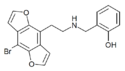
|
2C-B-DRAGONFLY-NBOH | N-(2-hydroxybenzyl)-1-(8-bromobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane | 1335331-45-7 |
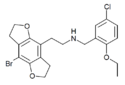
|
2C-B-FLY-NB2EtO5Cl [85] | N-(2-ethoxy-5-chlorobenzyl)-1-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b']difuran-4-yl)-2-aminoethane | |
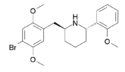
|
DMBMPP | (S,S)-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine | 1391499-52-7 |

|
2CLisaB | 2-[2-(4-bromo-2,5-dimethoxyphenyl)ethyl]-1,2,3,4-tetrahydroisoquinoline | |

|
2CBecca | 4-(4-bromo-2,5-dimethoxyphenyl)-1,2,3,4-tetrahydroisoquinoline | |

|
2CJP | 4-(4-bromo-2,5-dimethoxyphenyl)-2,3,4,5-tetrahydro-1H-2-benzazepine | |
| 25D-NM-NDEAOP | 3-[2-(2,5-dimethoxy-4-methylphenyl)ethyl-methylamino]-N,N-diethylpropanamide | ||
| Error creating thumbnail: | 25B-NAcPip | 2-{[2-(4-bromo-2,5-dimethoxyphenyl)ethyl]amino}-1-(piperidin-1-yl)ethanone | |
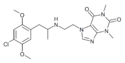
|
ZDCM-04 | 1,3-dimethyl-7-{2-[1-(2,5-dimethoxy-4-chlorophenyl)propan-2-ylamino]ethyl}purine-2,6-dione | |
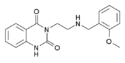
|
RH-34 | 3-[2-(2-methoxybenzylamino)ethyl]-1H-quinazoline-2,4-dione | 1028307-48-3 |

|
N-Benzyltryptamine (NBnT) | N-benzyltryptamine | 15741-79-4 |

|
4-HO-NBnT | 4-Hydroxy-N-benzyltryptamine | |

|
5-MeO-NBnT | 5-Methoxy-N-benzyltryptamine | 25100-31-6 |
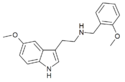
|
5-MeO-T-NBOMe[86] | N-(2-methoxybenzyl)-5-methoxytryptamine | 1335331-37-7 |
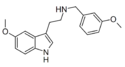
|
5MT-NB3OMe | N-(3-methoxybenzyl)-5-methoxytryptamine | 1648553-42-7 |

|
NEtPhOH-THPI | 3-(1-(2-hydroxyphenylethyl)-1,2,3,6-tetrahydropyridin-5-yl)-1H-indole | |

|
NBOMe-LAD (LSD-NBOMe) | N,N-diethyl-6-[(2-methoxyphenyl)methyl]-9,10-didehydroergoline-8β-carboxamide | ? |
See also
- Substituted methoxyphenethylamine
- 4-Substituted 2,5-dimethoxyphenethylamines (2Cs)
- 4-Substituted 2,5-dimethoxyamphetamines (DOx)
- 4-Substituted 2,5-dimethoxy-α-ethylphenethylamines (4Cs)
- List of miscellaneous 5-HT2A receptor agonists
Notes
- ↑ The potency of N-benzylphenethylamines via buccal, sublingual, or nasal absorption is 50- to 100-fold greater (by weight) than oral route compared to the parent 2C-x compounds.[30] Researchers hypothesize the low oral metabolic stability of N-benzylphenethylamines is likely causing the low bioavailability on the oral route, although the metabolic profile of this compounds remains unpredictable; therefore researchers state that the fatalities linked to these substances may partly be explained by differences in the metabolism between individuals.[30]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "DARK Classics in Chemical Neuroscience: NBOMes". ACS Chemical Neuroscience 11 (23): 3860–3869. December 2020. doi:10.1021/acschemneuro.9b00528. PMID 31657895.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens". Neuropharmacology of New Psychoactive Substances. Current Topics in Behavioral Neurosciences. 32. Springer. 18 January 2017. pp. 283–311. doi:10.1007/7854_2016_64. ISBN 978-3-319-52444-3. https://link.springer.com/chapter/10.1007/7854_2016_64.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 "NBOMes–Highly Potent and Toxic Alternatives of LSD". Frontiers in Neuroscience 14. 26 February 2020. doi:10.3389/fnins.2020.00078. PMID 32174803. "Recently, a new class of psychedelic compounds named NBOMe (or 25X-NBOMe) has appeared on the illegal drug market. NBOMes are analogs of the 2C family of phenethylamine drugs, originally synthesized by Alexander Shulgin, that contain a N-(2-methoxy)benzyl substituent. The most frequently reported drugs from this group are 25I-NBOMe, 25B-NBOMe, and 25C-NBOMe. NBOMe compounds are ultrapotent and highly efficacious agonists of serotonin 5-HT2A and 5-HT2C receptors (Ki values in low nanomolar range) with more than 1000-fold selectivity for 5-HT2A compared with 5-HT1A. They display higher affinity for 5-HT2A receptors than their 2C counterparts and have markedly lower affinity, potency, and efficacy at the 5-HT2B receptor compared to 5-HT2A or 5-HT2C.".
- ↑ "N-Benzylated derivatives of the hallucinogenic drugs mescaline and escaline as partial agonists at rat vascular 5-HT2A receptors". Naunyn-Schmiedeberg's Archives of Pharmacology 359: R29. 1999-01-01. http://bitnest.ca/external.php?id=%2502%257F%2505J%2516%251A%2509%2504%2504e%255C%25258%2522UV%2508%2507N%2501Q%2540i%251Ec%250B7kq.
- ↑ Heim R (February 28, 2010). Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts (Thesis) (in Deutsch). Berlin: Freie Univ. Retrieved 2013-05-10.
- ↑ Silva M (2009). Theoretical study of the interaction of agonists with the 5-HT2A receptor (Ph.D. thesis). Universität Regensburg.
- ↑ Hansen M (2011). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen.
- ↑ "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design 25 (1): 51–66. January 2011. doi:10.1007/s10822-010-9400-2. PMID 21088982. Bibcode: 2011JCAMD..25...51S.
- ↑ "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)". Neuropharmacology 99: 546–553. December 2015. doi:10.1016/j.neuropharm.2015.08.034. PMID 26318099. http://edoc.unibas.ch/56163/1/20170921163006_59c3cceeb8e5d.pdf.
- ↑ "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience 5 (3): 243–249. March 2014. doi:10.1021/cn400216u. PMID 24397362.
- ↑ "NBOMe Toxicity and Fatalities: A Review of the Literature". Transformative Medicine 1 (1): 12–18. 2022-03-30. doi:10.54299/tmed/msot8578. ISSN 2831-8978. https://scholarcommons.towerhealth.org/t-med/vol1/iss1/3.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 "NBOMe: new potent hallucinogens--pharmacology, analytical methods, toxicities, fatalities: a review". Eur Rev Med Pharmacol Sci 19 (17): 3270–3281. September 2015. PMID 26400534.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 "Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison With the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs". Front Psychiatry 13. 2022. doi:10.3389/fpsyt.2022.875722. PMID 35530025.
- ↑ 14.0 14.1 14.2 "2C-I-NBOMe, an "N-bomb" that kills with "Smiles". Toxicological and legislative aspects". Drug Chem Toxicol 38 (1): 113–119. January 2015. doi:10.3109/01480545.2014.911882. PMID 24785196.
- ↑ 15.0 15.1 15.2 15.3 "25C-NBOMe: preliminary data on pharmacology, psychoactive effects, and toxicity of a new potent and dangerous hallucinogenic drug". Biomed Res Int 2014. 2014. doi:10.1155/2014/734749. PMID 25105138.
- ↑ "Trips and neurotransmitters: Discovering principled patterns across 6850 hallucinogenic experiences". Sci Adv 8 (11). March 2022. doi:10.1126/sciadv.abl6989. PMID 35294242. Bibcode: 2022SciA....8L6989B.
- ↑ "Monoamine Transporter and Receptor Interaction Profiles in Vitro Predict Reported Human Doses of Novel Psychoactive Stimulants and Psychedelics". Int J Neuropsychopharmacol 21 (10): 926–931. October 2018. doi:10.1093/ijnp/pyy047. PMID 29850881.
- ↑ "Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species". Neuropharmacology 167. May 2020. doi:10.1016/j.neuropharm.2019.107933. PMID 31917152. PMC 9191653. http://usdbiology.com/cliff/Courses/Advanced%20Seminars%20in%20Neuroendocrinology/Serotonergic%20Psychedelics%2020/Halberstadt%2020%20Neuropharm%20potency%20of%20hallucinogens%20%20head-twitch.pdf. "Table 4 Human potency data for selected hallucinogens. [...]".
- ↑ "The use patterns of novel psychedelics: experiential fingerprints of substituted phenethylamines, tryptamines and lysergamides". Psychopharmacology (Berl) 239 (6): 1783–1796. June 2022. doi:10.1007/s00213-022-06142-4. PMID 35487983.
- ↑ 20.0 20.1 20.2 "A cluster of 25B-NBOH poisonings following exposure to powder sold as lysergic acid diethylamide (LSD)". Clinical Toxicology 60 (8): 966–969. 28 March 2022. doi:10.1080/15563650.2022.2053150. PMID 35343858. https://www.tandfonline.com/doi/abs/10.1080/15563650.2022.2053150.
- ↑ 21.0 21.1 21.2 21.3 "Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors". Biochemical Pharmacology 158: 27–34. December 2018. doi:10.1016/j.bcp.2018.09.024. PMID 30261175.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 "NBOMe Toxicity and Fatalities: A Review of the Literature". Transformative Medicine 1 (1): 12–18. 30 March 2022. doi:10.54299/tmed/msot8578. ISSN 2831-8978. https://scholarcommons.towerhealth.org/t-med/vol1/iss1/3/.
- ↑ 23.0 23.1 "Neurochemical and Behavioral Profiling in Male and Female Rats of the Psychedelic Agent 25I-NBOMe". Frontiers in Pharmacology 10. 12 December 2019. doi:10.3389/fphar.2019.01406. PMID 31915427.
- ↑ 24.0 24.1 24.2 "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)". Neuropharmacology 99: 546–553. December 2015. doi:10.1016/j.neuropharm.2015.08.034. ISSN 1873-7064. PMID 26318099. https://www.sciencedirect.com/science/article/abs/pii/S0028390815300794.
- ↑ 25.0 25.1 "Prevalence of use and acute toxicity associated with the use of NBOMe drugs". Clinical Toxicology 53 (2): 85–92. 6 February 2015. doi:10.3109/15563650.2015.1004179. PMID 25658166. https://www.tandfonline.com/doi/abs/10.3109/15563650.2015.1004179.
- ↑ "Toxic Leukoencephalopathy in a Teenager Caused by the Recreational Ingestion of 25I-NBOMe: A Case Report and Review of Literature". Journal of Medical Cases 8 (6): 174–179. 2017-06-05. doi:10.14740/jmc2811w. ISSN 1923-4163. https://www.journalmc.org/index.php/JMC/article/view/2811.
- ↑ "Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) MethylEthanamine Derivatives on Blotter Paper"]. Journal of Analytical Toxicology 39 (8): 617–623. 2015. doi:10.1093/jat/bkv073. PMID 26378135.
- ↑ "Death after 25C-NBOMe and 25H-NBOMe consumption". Forensic Science International 279: e1–e6. October 2017. doi:10.1016/j.forsciint.2017.08.028. PMID 28893436. https://www.sciencedirect.com/science/article/abs/pii/S0379073817303377.
- ↑ "Blunt Craniofacial Trauma as a Manifestation of Excited Delirium Caused by New Psychoactive Substances". Journal of Forensic Sciences 61 (6): 1546–1548. November 2016. doi:10.1111/1556-4029.13212. PMID 27723094. https://onlinelibrary.wiley.com/doi/abs/10.1111/1556-4029.13212.
- ↑ 30.0 30.1 "Correlating the Metabolic Stability of Psychedelic 5-HT2A Agonists with Anecdotal Reports of Human Oral Bioavailability". Neurochemical Research 39 (10): 2018–2023. 14 February 2014. doi:10.1007/s11064-014-1253-y. PMID 24519542. https://link.springer.com/article/10.1007/s11064-014-1253-y.
- ↑ "Analysis of 25 C NBOMe in Seized Blotters by HPTLC and GC–MS". Journal of Chromatographic Science 54 (7): 1153–1158. August 2016. doi:10.1093/chromsci/bmw095. PMID 27406128. PMC 4941995. https://academic.oup.com/chromsci/article/54/7/1153/2754859.
- ↑ "Pharmacology and toxicology of N-Benzyl-phenylethylamines (25X-NBOMe) hallucinogens". Novel Psychoactive Substances: Classification, Pharmacology and Toxicology (2 ed.). Academic Press. September 2021. pp. 279–300. doi:10.1016/B978-0-12-818788-3.00008-5. ISBN 978-0-12-818788-3. https://www.sciencedirect.com/science/article/abs/pii/B9780128187883000085.
- ↑ "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications". Circulation 102 (23): 2836–41. Dec 2000. doi:10.1161/01.CIR.102.23.2836. PMID 11104741.
- ↑ "Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine". Molecular Pharmacology 57 (1): 75–81. Jan 2000. doi:10.1016/S0026-895X(24)26444-0. PMID 10617681.
- ↑ "Drugs and valvular heart disease". The New England Journal of Medicine 356 (1): 6–9. Jan 2007. doi:10.1056/NEJMp068265. PMID 17202450. https://www.nejm.org/doi/full/10.1056/NEJMp068265.
- ↑ "25C-NBOMe, a Novel Designer Psychedelic, Induces Neurotoxicity 50 Times More Potent Than Methamphetamine In Vitro". Neurotoxicity Research 35 (4): 993–998. 26 February 2019. doi:10.1007/s12640-019-0012-x. PMID 30806983. https://link.springer.com/article/10.1007/s12640-019-0012-x.
- ↑ "Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity". Toxicology Reports 8: 315–323. 2021. doi:10.1016/j.toxrep.2021.01.010. ISSN 2214-7500. PMID 33598409. Bibcode: 2021ToxR....8..315A.
- ↑ 38.0 38.1 38.2 "2C or not 2C: phenethylamine designer drug review". J Med Toxicol 9 (2): 172–178. June 2013. doi:10.1007/s13181-013-0295-x. PMID 23494844.
- ↑ 39.0 39.1 39.2 "Identification of monoamine oxidase and cytochrome P450 isoenzymes involved in the deamination of phenethylamine-derived designer drugs (2C-series)". Biochem Pharmacol 73 (2): 287–297. January 2007. doi:10.1016/j.bcp.2006.09.022. PMID 17067556.
- ↑ 40.0 40.1 "Drug-drug interactions involving classic psychedelics: A systematic review". J Psychopharmacol 38 (1): 3–18. January 2024. doi:10.1177/02698811231211219. PMID 37982394.
- ↑ 41.0 41.1 "Characterization of the hepatic cytochrome P450 enzymes involved in the metabolism of 25I-NBOMe and 25I-NBOH". Drug Test Anal 9 (5): 671–679. May 2017. doi:10.1002/dta.2031. PMID 27400739.
- ↑ 42.0 42.1 42.2 "Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors". Biochem Pharmacol 158: 27–34. December 2018. doi:10.1016/j.bcp.2018.09.024. PMID 30261175.
- ↑ 43.0 43.1 "Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs)". Neuropharmacology 99: 546–553. December 2015. doi:10.1016/j.neuropharm.2015.08.034. PMID 26318099. http://edoc.unibas.ch/56163/1/20170921163006_59c3cceeb8e5d.pdf.
- ↑ 44.0 44.1 "Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential". Nat Commun 14 (1). December 2023. doi:10.1038/s41467-023-44016-1. PMID 38102107. Bibcode: 2023NatCo..14.8221W.
- ↑ "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1". J Pharmacol Exp Ther 357 (1): 134–144. April 2016. doi:10.1124/jpet.115.229765. PMID 26791601. https://d1wqtxts1xzle7.cloudfront.net/74120533/eae6c6e62565b82d46b4d111bbea0f77b9c2-libre.pdf?1635931703=&response-content-disposition=inline%3B+filename%3DIn_Vitro_Characterization_of_Psychoactiv.pdf&Expires=1746838268&Signature=Sy4fJ90yUhxs68314NxYsW5PAaNrBGePRu35WRR4PIF-3YC7Z~sLdnCn5wfqqbLg9bDEGdt~oW55ugMP3D3jgA0BoRI~~GOb0NQOwrtfUEQK1PQs1uuN9qg5Y1ct8z5NsABm44RgtukkwRMdU6fO7OlfIsQ68hOiFk129Ll7UYqldxD2f1xhE2fTTfsxSpb8cMCJzHn7-ItqLdwnAUPFK7WggDIjmY1kCnaHLwIxMwdJCAq8L6DYzSTg7pZkbR8qlou~GXbTPQt~gYpyZTJp5hgW-7V6K5wLlQ7Z2xE7B0f9wEfuc1W1QNafg125Tr-vvAe4LEGKXV58bnn1bpfWKw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
- ↑ 46.0 46.1 46.2 "Toxicodynamic insights of 2C and NBOMe drugs - Is there abuse potential?". Toxicol Rep 14. June 2025. doi:10.1016/j.toxrep.2025.101890. PMID 39867514. Bibcode: 2025ToxR...1401890G.
- ↑ 47.0 47.1 47.2 "25B-NBOMe, a novel N-2-methoxybenzyl-phenethylamine (NBOMe) derivative, may induce rewarding and reinforcing effects via a dopaminergic mechanism: Evidence of abuse potential". Addict Biol 25 (6). November 2020. doi:10.1111/adb.12850. PMID 31749223.
- ↑ 48.0 48.1 48.2 "A novel designer drug, 25N-NBOMe, exhibits abuse potential via the dopaminergic system in rodents". Brain Res Bull 152: 19–26. October 2019. doi:10.1016/j.brainresbull.2019.07.002. PMID 31279579.
- ↑ 49.0 49.1 "New designer phenethylamines 2C-C and 2C-P have abuse potential and induce neurotoxicity in rodents". Arch Toxicol 95 (4): 1413–1429. April 2021. doi:10.1007/s00204-021-02980-x. PMID 33515270. Bibcode: 2021ArTox..95.1413K. "25N-NBOMe and other 2C drug derivatives similarly increased p-DAT levels in the NAc and striatum of mice (Seo et al. 2019). [...] increased p-DAT levels lead to an increase in dopamine release, which contribute to elevated dopamine levels.".
- ↑ "Tolerance to neurochemical and behavioral effects of the hallucinogen 25I-NBOMe". Psychopharmacology (Berl) 238 (8): 2349–2364. August 2021. doi:10.1007/s00213-021-05860-5. PMID 34032876.
- ↑ Jenkins, Amanda J.; Gates, Madeleine J. (2020). "Hallucinogens and Psychedelics". Principles of Forensic Toxicology. Cham: Springer International Publishing. pp. 467–489. doi:10.1007/978-3-030-42917-1_26. ISBN 978-3-030-42916-4. http://link.springer.com/10.1007/978-3-030-42917-1_26. Retrieved 30 June 2025. "No human pharmacological data exist [for NBOMe compounds], although metabolism has been investigated in vitro."
- ↑ Michael Robert Braden (2007). "Towards a biophysical understanding of hallucinogen action". Dissertation: 1–176. https://docs.lib.purdue.edu/dissertations/AAI3287241/.
- ↑ "Structure-activity relationships of serotonin 5-HT2A agonists". Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1 (5): 559–579. 2012. doi:10.1002/wmts.42.
- ↑ "5-HT2A/5-HT2C Receptor Pharmacology and Intrinsic Clearance of N-Benzylphenethylamines Modified at the Primary Site of Metabolism". ACS Chemical Neuroscience 7 (11): 1614–1619. November 2016. doi:10.1021/acschemneuro.6b00265. PMID 27564969.
- ↑ "In vivo evaluation of [18FFECIMBI-36, an agonist 5-HT2A/2C receptor PET radioligand in nonhuman primate"]. Bioorganic & Medicinal Chemistry Letters 27 (1): 21–23. January 2017. doi:10.1016/j.bmcl.2016.11.043. PMID 27889455.
- ↑ "Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands". ACS Chemical Neuroscience 4 (1): 96–109. January 2013. doi:10.1021/cn3000668. PMID 23336049.
- ↑ 57.0 57.1 "Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens". Neuropharmacology of New Psychoactive Substances (NPS).. Current Topics in Behavioral Neurosciences. 32. Cham: Springer. 2017. pp. 283–311. doi:10.1007/7854_2016_64. ISBN 978-3-319-52444-3.
- ↑ 58.0 58.1 "Influence of amine substituents on 5-HT2A versus 5-HT2C binding of phenylalkyl- and indolylalkylamines". Journal of Medicinal Chemistry 37 (13): 1929–1935. June 1994. doi:10.1021/jm00039a004. PMID 8027974.
- ↑ "P 8.10. Congeners of 3-(2-benzylaminoethyl)-2, 4-quinazolinedione: Partial agonists for rat vascular 5-HT2A receptors.". Naunyn-Schmiedebergs Archives of Pharmacology 358 (1): R105. January 1998. https://bitnest.netfirms.com/external/N-S.Arch.Pharmacol/358.S1.R105.
- ↑ "Abstracts: 331: Characterization of the Partial Agonism of Ergoline Reverse Esters, Indolyltetrahydropyridines, and Quinazolinediones at 5-HT2A receptors in Rat Tail Artery". Naunyn-Schmiedeberg's Archives of Pharmacology 353 (S4): R1–R166 (R90–R91). 1996. doi:10.1007/BF00625102. ISSN 0028-1298. http://link.springer.com/10.1007/BF00625102. "The aim of the study was to characterize the partial agonism of congeners of well-established 5-HT2A receptor ligands, identified in a series of ergot alkaloids (so-called ergoline reverse esters with lysergol and dihydrolysergol-I as alcoholic component), indolyltetrahydropyridines (RU 24969 and two derivatives), and quinazolinediones (derivatives of ketanserin), at 5-HT2A receptors in rat tail artery. [...] Quinazolinediones (derivatives of ketanserin) showed weak agonist activity (pKp = 3.83 - 4.66, α = 0.17 - 0,46) and antagonized contractile responses to 5-HT with calculated pKp values of 3.52 - 5.12.".
- ↑ "Development of highly potent partial agonists and chiral antagonists as tools for the study of 5-HT2A-receptor mediated function". Naunyn-Schmiedeberg's Archives of Pharmacology 365 (1 Suppl): R21–R40. 2002. doi:10.1007/s00210-002-0604-4. https://bitnest.netfirms.com/external/N-S.Arch.Pharmacol/365.S1.R29.
- ↑ Pertz, H. H.; Rheineck, A.; Elz, S. (1999). "N-Benzylated derivatives of the hallucinogenic drugs mescaline and escaline as partial agonists at rat vascular 5-HT2A receptors.". Naunyn-Schmiedeberg's Arch Pharmacol 359 (Suppl 3): R29.
- ↑ Ratzeburg, K.; Heim, R.; Mahboobi, S.; Henatsch, J.; Pertz, H. H.; Elz, S. (March 2003). "Potent partial 5-HT2A-receptor agonism of phenylethan-amines related to mescaline in the rat tail artery model.". Naunyn-Schmiedebergs Archives of Pharmacology 167: R31.
- ↑ 64.0 64.1 Chemistry and Structure-Activity Relationships of Psychedelics. Current Topics in Behavioral Neurosciences. 36. 2018. pp. 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. https://bitnest.netfirms.com/external/10.1007/7854_2017_475. "Although the most active tryptamine hallucinogens are N,N-dialkylated, the phenethylamines generally cannot tolerate even a single N-substitution. Even small groups such as methyl or ethyl (see Table 2) abolish their hallucinogenic activity. It was quite remarkable, therefore, when Heim and coworkers reported that N-benzyl groups afforded compounds with remarkable affinity and potency (Heim et al. 1999; Elz et al. 2002; Heim 2003). An oxygen atom at the ortho position of the N-benzyl group enhanced activity further (Braden et al. 2006)."
- ↑ Heim, R.; Pertz, H. H.; Elz, S. (1999). "Preparation and in vitro pharmacology of novel secondary amine-type 5-HT2A receptor agonists: from submillimolar to subnanomolar activity". Arch. Pharm. Pharm. Med. Chem 332 (34). https://bitnest.netfirms.com/external/Arch.Pharm.Pharm.Med.Chem/331.S1.34.
- ↑ "39. Novel Extremely Potent Partial 5-HT2A-Receptor Agonists: Successful Application of a New Structure-Activity Concept". Arch. Pharm. Pharm. Med. Chem. 333 (Suppl 1): 1–40 (18). March 2000. ISSN 0365-6233.
- ↑ "B 1.11. N-Benzylated phenylethanamines are highly potent partial agonists at 5-HT2A receptors". Arch. Pharm. Pharm. Med. Chem 333 (Suppl 2): 1–84 (30). 2000.
- ↑ Heim, R.; Pertz, H. H.; Elz, S. (2000). "Partial 5-HT2A-receptor agonists of the phenylethanamine series: effect of a trifluoromethyl substituent". Arch. Pharm. Pharm. Med. Chem 333 (45). https://bitnest.netfirms.com/external/Arch.Pharm.Pharm.Med.Chem/333.S2.45.
- ↑ 69.0 69.1 "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts." (in German). diss.fu-berlin.de. 25 March 2003. http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221.
- ↑ Ettrup, A. (2010). Serotonin receptor studies in the pig brain: pharmacological intervention and positron emission tomography tracer development (PhD thesis). Faculty of Health Sciences, University of Copenhagen.
- ↑ Ettrup, Anders; Hansen, Martin; Santini, Martin A.; Paine, James; Gillings, Nic; Palner, Mikael; Lehel, Szabolcs; Herth, Matthias M. et al. (2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging 38 (4): 681–693. doi:10.1007/s00259-010-1686-8. ISSN 1619-7089. PMID 21174090.
- ↑ "Prevalence of use and acute toxicity associated with the use of NBOMe drugs". Clin Toxicol (Phila) 53 (2): 85–92. February 2015. doi:10.3109/15563650.2015.1004179. PMID 25658166.
- ↑ Morgans, Julian (8 February 2017). "NBOMe in Australia: Everything We Know About the Drug and Why it's Killing People". https://www.vice.com/en/article/nbome-in-australia-everything-we-know-about-what-it-is-and-why-its-killing-people/.
- ↑ "The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample". J Psychopharmacol 28 (8): 780–788. August 2014. doi:10.1177/0269881114523866. PMID 24569095.
- ↑ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014" (in en). http://www.legislation.gov.uk/uksi/2014/1106/made.
- ↑ "Psychoactive Substances Act 2016" (in en). http://www.legislation.gov.uk/ukpga/2016/2/contents/enacted.
- ↑ Patentscope. Wallach J, et al. Selective, Partial and Arrestin-Biased 5-HT2A Agonists with Utility in Various Disorders. Patent WO 2022/241006. Retrieved 2025-05-12
- ↑ "Identification of 5-HT2A Receptor Signaling Pathways Responsible for Psychedelic Potential". bioRxiv. 2023. doi:10.1101/2023.07.29.551106.
- ↑ "Synthesis and Structure-Activity Relationships of 2,5-Dimethoxy-4-Substituted Phenethylamines and the Discovery of CYB210010: A Potent, Orally Bioavailable and Long-Acting Serotonin 5-HT2 Receptor Agonist". Journal of Medicinal Chemistry 67 (8): 6144–6188. April 2024. doi:10.1021/acs.jmedchem.3c01961. PMID 38593423.
- ↑ Patentscope. Kruegel AC. Phenalkylamines and Methods of Making and Using the Same. Patent WO 2022/192781. Retrieved 2025-05-12
- ↑ Phenethylamine Von der Struktur zur Funktion. Nachtschatten Verlag AG. 2013. p. 843. ISBN 978-3-03788-700-4.
- ↑ "Prodrugs of New Psychoactive Substances (NPS): A New Challenge". Journal of Forensic Sciences 65 (3): 913–920. May 2020. doi:10.1111/1556-4029.14268. PMID 31943218. https://researchonline.ljmu.ac.uk/id/eprint/11926/1/JOFS-19-562.R1_accepted_uncorrected.pdf.
- ↑ "PiHKAL·info". 1 April 2025. https://isomerdesign.com/pihkal/explore/1074.
- ↑ "The Serotonin 5-HT2A Receptor as an Evolving Neurotherapeutic Target". Medicinal Chemistry Reviews 58 (3): 53–81. 2023. doi:10.1021/mc-2023-vol58.ch03.
- ↑ "In vitro toxicokinetics and analytical toxicology of three novel NBOMe derivatives: Phase I and II metabolism, plasma protein binding, and detectability in standard urine screening approaches studied by means of hyphenated mass spectrometry". Forensic Toxicology 38: 141–159. 2020. doi:10.1007/s11419-019-00498-7. http://researchonline.ljmu.ac.uk/id/eprint/11258/1/FOTO-D-19-00080.R2_accepted.pdf.
- ↑ "N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues". ACS Chemical Neuroscience 6 (7): 1165–1175. July 2015. doi:10.1021/cn500292d. PMID 25547199.
External links
- VICE In-house Chemist Hamilton Morris on the Dangers of the NBOMe Hallucinogen - VICE - YouTube
- What Are N-Bombs? (25-I-NBOMe) — Avoid This Psychedelic - Tripsitter
- Unmasking Fake Acid: The Dangers of 25I-NBOMe Disguised as LSD - Psychedelic Spotlight
Template:Chemical classes of psychoactive drugs
 |
