Biology:Protein splicing
Protein splicing is an intramolecular reaction of a particular protein in which an internal protein segment (called an intein) is removed from a precursor protein with a ligation of C-terminal and N-terminal external proteins (called exteins) on both sides. The splicing junction of the precursor protein is mainly a cysteine or a serine, which are amino acids containing a nucleophilic side chain. The protein splicing reactions which are known now do not require exogenous cofactors or energy sources such as adenosine triphosphate (ATP) or guanosine triphosphate (GTP). Normally, splicing is associated only with pre-mRNA splicing. This precursor protein contains three segments—an N-extein followed by the intein followed by a C-extein. After splicing has taken place, the resulting protein contains the N-extein linked to the C-extein; this splicing product is also termed an extein.
History
The first intein was discovered in 1988 through sequence comparison between the Neurospora crassa[1] and carrot[2] vacuolar ATPase (without intein) and the homologous gene in yeast (with intein) that was first described as a putative calcium ion transporter.[3] In 1990 Hirata et al.[4] demonstrated that the extra sequence in the yeast gene was transcribed into mRNA and removed itself from the host protein only after translation. Since then, inteins have been found in all three domains of life (eukaryotes, bacteria, and archaea) and in viruses.
Protein splicing was unanticipated and its mechanisms were discovered by two groups (Anraku [5] and Stevens[6]) in 1990. They both discovered a Saccharomyces cerevisiae VMA1 in a precursor of a vacuolar H+-ATPase enzyme. The amino acid sequence of the N- and C-termini corresponded to 70% DNA sequence of that of a vacuolar H+-ATPase from other organisms, while the amino acid sequence of the central position corresponded to 30% of the total DNA sequence of the yeast HO nuclease.
Many genes have unrelated intein-coding segments inserted at different positions. For these and other reasons, inteins (or more properly, the gene segments coding for inteins) are sometimes called selfish genetic elements, but it may be more accurate to call them parasitic. According to the gene centered view of evolution, most genes are "selfish" only insofar as to compete with other genes or alleles but usually they fulfill a function for the organisms, whereas "parasitic genetic elements", at least initially, do not make a positive contribution to the fitness of the organism.[7][8]
As of December 2019, the UniProtKB database contains 188 entries manually annotated as inteins, ranging from just tens of amino acid residues to thousands.[9] The first intein was found encoded within the VMA gene of Saccharomyces cerevisiae. They were later found in fungi (ascomycetes, basidiomycetes, zygomycetes and chytrids) and in diverse proteins as well. A protein distantly related to known inteins containing protein, but closely related to metazoan hedgehog proteins, has been described to have the intein sequence from Glomeromycota. Many of the newly described inteins contain homing endonucleases and some of these are apparently active.[10] The abundance of intein in fungi indicates lateral transfer of intein-containing genes. While in eubacteria and archaea, there are 289 and 182 currently known inteins. Not surprisingly, most intein in eubacteria and archaea are found to be inserted into nucleic acid metabolic protein, like fungi.[10]
Inteins vary greatly, but many of the same intein-containing proteins are found in a number of species. For example, pre-mRNA processing factor 8 (Prp8) protein, instrumental in the spliceosome, has seven different intein insertion sites across eukaryotic species.[11] Intein-containing Prp8 is most commonly found in fungi, but is also seen in Amoebozoa, Chlorophyta, Capsaspora, and Choanoflagellida. Many mycobacteria contain inteins within DnaB (bacterial replicative helicase), RecA (bacterial DNA recombinase), and SufB (FeS cluster assembly protein).[12][13] There is remarkable variety within the structure and number of DnaB inteins, both within the mycobacterium genus and beyond. Interestingly, intein-containing DnaB is also found in the chloroplasts of algae.[14] Intein-containing proteins found in archaea include RadA (RecA homolog), RFC, PolB, RNR.[15] Many of the same intein-containing proteins (or their homologs) are found in two or even all three domains of life. Inteins are also seen in the proteomes encoded by bacteriophages and eukaryotic viruses. Viruses may have been involved as vectors of intein distribution across the wide variety of intein containing organisms.[15]
Mechanism
This section is missing information about Conserved A, B, F, G blocks . (November 2023) |
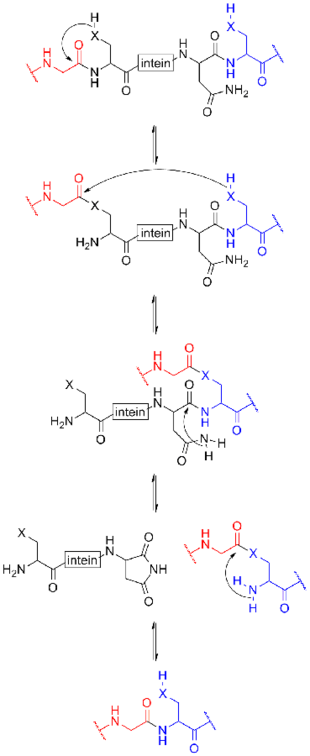
The process for class 1 inteins begins with an N-O or N-S shift when the side chain of the first residue (a serine, threonine, or cysteine) of the intein portion of the precursor protein nucleophilically attacks the peptide bond of the residue immediately upstream (that is, the final residue of the N-extein) to form a linear ester (or thioester) intermediate. A transesterification occurs when the side chain of the first residue of the C-extein attacks the newly formed (thio)ester to free the N-terminal end of the intein. This forms a branched intermediate in which the N-extein and C-extein are attached, albeit not through a peptide bond. The last residue of the intein is always an asparagine (Asn), and the amide nitrogen atom of this side chain cleaves apart the peptide bond between the intein and the C-extein, resulting in a free intein segment with a terminal cyclic imide. Finally, the free amino group of the C-extein now attacks the (thio)ester linking the N- and C-exteins together. An O-N or S-N shift produces a peptide bond and the functional, ligated protein.[16]
Class 2 inteins have no nucleophilic first side chain, only an alanine. Instead, the reaction starts directly with a nucleophilic displacement, with the first residue of the C-extein atticking the peptide carboxyl on the final residue of the N-extein. The rest proceeds as usual, starting with Asn turning into a cyclic imide.[17]
Class 3 inteins have no nucleophilic first side chain, only an alanine, yet they have an internal noncontiguous "WCT" motif. The internal C (cysteine) residue attacks the peptide carboxyl on the final residue of the N-extein (nucleophilic displacement). Transesterification occurs when the first residue of the C-extein attacks the newly formed thioester. The rest proceeds as usual.[18]
The mechanism for the splicing effect is a naturally occurring analogy to the technique for chemically generating medium-sized proteins called native chemical ligation.
Intein
An intein is a segment of a protein that is able to excise itself and join the remaining portions (the exteins) with a peptide bond during protein splicing.[19] Inteins have also been called protein introns, by analogy with (RNA) introns.
Naming conventions
The first part of an intein name is based on the scientific name of the organism in which it is found, and the second part is based on the name of the corresponding gene or extein. For example, the intein found in Thermoplasma acidophilum and associated with Vacuolar ATPase subunit A (VMA) is called "Tac VMA".
Normally, as in this example, just three letters suffice to specify the organism, but there are variations. For example, additional letters may be added to indicate a strain. If more than one intein is encoded in the corresponding gene, the inteins are given a numerical suffix starting from 5′ to 3′ or in order of their identification (for example, "Msm dnaB-1").
The segment of the gene that encodes the intein is usually given the same name as the intein, but to avoid confusion the name of the intein proper is usually capitalized (e.g., Pfu RIR1-1), whereas the name of the corresponding gene segment is italicized (e.g., Pfu rir1-1). A different disambiguating convention is to place a lowercase "i" after the source protein name, e.g. "Msm DnaBi1".[20]
Types of inteins
Inteins can be classified on many criteria.
- Based on how they splice themselves out, they can be classified into cis-splicing (which means that they splice themselves out) or trans-splicing (which means they need outside help). Most studied inteins are cis-splicing. Split inteins (see below) usually involves two halves helping each other out, so they are trans-splicing.[17]
- Based on whether they contain the endonuclease domain. Ones that have an endonuclease domain is called a "maxi-intein", otherwise a "mini-intein".[17]
- Based on their splicing mechanism, which can be partially[18] inferred based on the sequence. Class 1 intein is the most-studied type and are marked by a cysteine or serine as the first residue. Class 2 intein, or "alanine intein" has alanine as the first residue and no WCT motif. Class 3 intein has alanine as the first residue and a noncontiguous "WCT" motif.[17] It has also been proposed that inteins that start with a serine and contain a "WCT" motif should be also classed as class 3.[18]
Full and mini inteins
Inteins can contain a homing endonuclease gene (HEG) domain in addition to the splicing domains. This domain is responsible for the spread of the intein by cleaving DNA at an intein-free allele on the homologous chromosome, triggering the DNA double-stranded break repair (DSBR) system, which then repairs the break, thus copying the intein-coding DNA into a previously intein-free site.[17] The HEG domain is not necessary for intein splicing, and so it can be lost, forming a minimal, or mini, intein. Several studies have demonstrated the modular nature of inteins by adding or removing HEG domains and determining the activity of the new construct.[citation needed]
Split inteins
Sometimes, the intein of the precursor protein comes from two genes. In this case, the intein is said to be a split intein. For example, in cyanobacteria, DnaE, the catalytic subunit α of DNA polymerase III, is encoded by two separate genes, dnaE-n and dnaE-c. The dnaE-n product consists of an N-extein sequence followed by a 123-AA intein sequence, whereas the dnaE-c product consists of a 36-AA intein sequence followed by a C-extein sequence.[21]
Applications in biotechnology
Inteins are very efficient at protein splicing, and they have accordingly found an important role in biotechnology. There are more than 200 inteins identified to date; sizes range from 100–800 AAs. Inteins have been engineered for particular applications such as protein semisynthesis[22] and the selective labeling of protein segments, which is useful for NMR studies of large proteins.[23]
Pharmaceutical inhibition of intein excision may be a useful tool for drug development; the protein that contains the intein will not carry out its normal function if the intein does not excise, since its structure will be disrupted.
It has been suggested that inteins could prove useful for achieving allotopic expression of certain highly hydrophobic proteins normally encoded by the mitochondrial genome, for example in gene therapy.[24] The hydrophobicity of these proteins is an obstacle to their import into mitochondria. Therefore, the insertion of a non-hydrophobic intein may allow this import to proceed. Excision of the intein after import would then restore the protein to wild-type.
Affinity tags have been widely used to purify recombinant proteins, as they allow the accumulation of recombinant protein with little impurities. However, the affinity tag must be removed by proteases in the final purification step. The extra proteolysis step raises the problems of protease specificity in removing affinity tags from recombinant protein, and the removal of the digestion product. This problem can be avoided by fusing an affinity tag to self-cleavable inteins in a controlled environment. The first generation of expression vectors of this kind used modified Saccharomyces cerevisiae VMA (Sce VMA) intein. Chong et al.[25] used a chitin binding domain (CBD) from Bacillus circulans as an affinity tag, and fused this tag with a modified Sce VMA intein. The modified intein undergoes a self-cleavage reaction at its N-terminal peptide linkage with 1,4-dithiothreitol (DTT), β-mercaptoethanol (β-ME), or cystine at low temperatures over a broad pH range. After expressing the recombinant protein, the cell homogenate is passed through the column containing chitin. This allows the CBD of the chimeric protein to bind to the column. Furthermore, when the temperature is lowered and the molecules described above pass through the column, the chimeric protein undergoes self-splicing and only the target protein is eluted. This novel technique eliminates the need for a proteolysis step, and modified Sce VMA stays in column attached to chitin through CBD.[25]
Recently inteins have been used to purify proteins based on self aggregating peptides. Elastin-like polypeptides (ELPs) are a useful tool in biotechnology. Fused with target protein, they tend to form aggregates inside the cells.[26] This eliminates the chromatographic step needed in protein purification. The ELP tags have been used in the fusion protein of intein, so that the aggregates can be isolated without chromatography (by centrifugation) and then intein and tag can be cleaved in controlled manner to release the target protein into solution. This protein isolation can be done using continuous media flow, yielding high amounts of protein, making this process more economically efficient than conventional methods.[26] Another group of researchers used smaller self aggregating tags to isolate target protein. Small amphipathic peptides 18A and ELK16 (figure 5) were used to form self cleaving aggregating protein.[27]
Applications in Antimicrobial Development
Over the last twenty years, there has been increasing interest in leveraging inteins for antimicrobial applications.[12] Intein splicing is found exclusively in unicellular organisms, with a particularly high abundance in pathogenic microorganisms.[28] Furthermore, inteins are commonly found within housekeeping proteins and/or proteins involved in the survival of the organism within a human host. Post-translational intein removal is necessary for the protein to properly fold and function. For example, Gaëlle Huet et al. demonstrated that in Mycobacterium tuberculosis, unspliced SufB prevents the formation of the SufBCD complex, a component of the SUF machinery.[29] As such, the inhibition of intein splicing may serve as a powerful platform for the development of antimicrobials.
Current research on intein splicing inhibitors has focused on developing antimycobacterials (M. tb. has three intein-containing proteins), as well as agents active against pathogenic fungi Cryptococcus and Aspergillus.[13] Cisplatin and similar platinum-containing compounds inhibit splicing of the M. tb. RecA intein through coordinating to catalytic residues.[30] Divalent cations, such as copper (II) and zinc (II) ions, function similarly to reversibly inhibit splicing.[12] However, neither of these methods are currently suitable for an effective and safe antibiotic. The fungal Prp8 intein is also inhibited by divalent cations and cisplatin through interfering with the catalytic Cys1 residue.[12] In 2021, Li et al. showed that small molecule inhibitors of Prp8 intein splicing were selective and effective at slowing the growth of C. neoformans and C. gattii, providing exciting evidence for the antimicrobial potential of intein splicing inhibitors.[31]
See also
References
- ↑ Bowman, EJ; Tenney, K; Bowman, BJ (Oct 1988). "Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases". J Biol Chem 263 (28): 13994–4001. doi:10.1016/S0021-9258(18)68175-X. PMID 2971651.
- ↑ Zimniak, L; Dittrich, P; Gogarten, JP; Kibak, H; Taiz, L (Jul 1988). "The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases". J Biol Chem 263 (19): 9102–12. doi:10.1016/S0021-9258(19)76514-4. PMID 2897965.
- ↑ Shih, CK; Wagner, R; Feinstein, S; Kanik-Ennulat, C; Neff, N (Aug 1988). "A dominant trifluoperazine resistance gene from Saccharomyces cerevisiae has homology with F0F1 ATP synthase and confers calcium-sensitive growth". Mol Cell Biol 8 (8): 3094–103. doi:10.1128/mcb.8.8.3094. PMID 2905423.
- ↑ Hirata, R; Ohsumk, Y; Nakano, A; Kawasaki, H; Suzuki, K; Anraku, Y (Apr 1990). "Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae". J Biol Chem 265 (12): 6726–33. doi:10.1016/S0021-9258(19)39210-5. PMID 2139027.
- ↑ "Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae". J. Biol. Chem. 265 (12): 6726–33. April 1990. doi:10.1016/S0021-9258(19)39210-5. PMID 2139027.
- ↑ "Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H(+)-adenosine triphosphatase". Science 250 (4981): 651–7. November 1990. doi:10.1126/science.2146742. PMID 2146742. Bibcode: 1990Sci...250..651K.
- ↑ Swithers, Kristen S.; Soucy, Shannon M.; Gogarten, J. Peter (2012). "The Role of Reticulate Evolution in Creating Innovation and Complexity". International Journal of Evolutionary Biology 2012: 1–10. doi:10.1155/2012/418964. ISSN 2090-8032. PMID 22844638.
- ↑ Dawkins, Richard (1976). The Selfish Gene. Oxford University Press.
- ↑ "UniProt: the universal protein knowledgebase". Nucleic Acids Research 45 (D1): D158–D169. 2016-11-29. doi:10.1093/nar/gkw1099. ISSN 0305-1048. PMID 27899622. PMC 5210571. http://dx.doi.org/10.1093/nar/gkw1099.
- ↑ 10.0 10.1 Perler, F. B. (2002). "InBase: the Intein Database". Nucleic Acids Research 30 (1): 383–384. doi:10.1093/nar/30.1.383. ISSN 1362-4962. PMID 11752343.
- ↑ Green, Cathleen M.; Li, Zhong; Smith, Aaron D.; Novikova, Olga; Bacot-Davis, Valjean R.; Gao, Fengshan; Hu, Saiyang; Banavali, Nilesh K. et al. (2019-10-10). "Spliceosomal Prp8 intein at the crossroads of protein and RNA splicing" (in en). PLOS Biology 17 (10): e3000104. doi:10.1371/journal.pbio.3000104. ISSN 1545-7885. PMID 31600193.
- ↑ 12.0 12.1 12.2 12.3 Tharappel, Anil Mathew; Li, Zhong; Li, Hongmin (2022). "Inteins as Drug Targets and Therapeutic Tools". Frontiers in Molecular Biosciences 9: 821146. doi:10.3389/fmolb.2022.821146. ISSN 2296-889X. PMID 35211511.
- ↑ 13.0 13.1 Wall, Diana A.; Tarrant, Seanan P.; Wang, Chunyu; Mills, Kenneth V.; Lennon, Christopher W. (2021). "Intein Inhibitors as Novel Antimicrobials: Protein Splicing in Human Pathogens, Screening Methods, and Off-Target Considerations". Frontiers in Molecular Biosciences 8: 752824. doi:10.3389/fmolb.2021.752824. ISSN 2296-889X. PMID 34692773.
- ↑ Green, Cathleen M.; Novikova, Olga; Belfort, Marlene (2018-01-24). "The dynamic intein landscape of eukaryotes". Mobile DNA 9 (1): 4. doi:10.1186/s13100-018-0111-x. ISSN 1759-8753. PMID 29416568.
- ↑ 15.0 15.1 Novikova, Olga; Topilina, Natalya; Belfort, Marlene (May 2014). "Enigmatic Distribution, Evolution, and Function of Inteins". Journal of Biological Chemistry 289 (21): 14490–14497. doi:10.1074/jbc.r114.548255. ISSN 0021-9258. PMID 24695741.
- ↑ "Dissecting the chemistry of protein splicing and its applications". Angew Chem Int Ed Engl 39 (3): 450–66. 2000. doi:10.1002/(sici)1521-3773(20000204)39:3<450::aid-anie450>3.3.co;2-6. PMID 10671234.
- ↑ 17.0 17.1 17.2 17.3 17.4 Nanda, A; Nasker, SS; Mehra, A; Panda, S; Nayak, S (16 December 2020). "Inteins in Science: Evolution to Application.". Microorganisms 8 (12): 2004. doi:10.3390/microorganisms8122004. PMID 33339089.
- ↑ 18.0 18.1 18.2 Tori, K; Perler, FB (April 2011). "Expanding the definition of class 3 inteins and their proposed phage origin.". Journal of Bacteriology 193 (8): 2035–41. doi:10.1128/JB.01407-10. PMID 21317331.
- ↑ Anraku, Y; Mizutani, R; Satow, Y (2005). "Protein splicing: its discovery and structural insight into novel chemical mechanisms". IUBMB Life 57 (8): 563–74. doi:10.1080/15216540500215499. PMID 16118114.
- ↑ Kelley, Danielle S.; Lennon, Christopher W.; Li, Zhong; Miller, Michael R.; Banavali, Nilesh K.; Li, Hongmin; Belfort, Marlene (19 October 2018). "Mycobacterial DnaB helicase intein as oxidative stress sensor". Nature Communications 9 (1): 4363. doi:10.1038/s41467-018-06554-x. PMID 30341292. Bibcode: 2018NatCo...9.4363K.
- ↑ Wu, H.; Hu, Z.; Liu, X. Q. (1998). "Protein trans-splicing by a split intein encoded in a split DnaE gene of Synechocystis sp. PCC6803". Proceedings of the National Academy of Sciences of the United States of America 95 (16): 9226–9231. doi:10.1073/pnas.95.16.9226. PMID 9689062. Bibcode: 1998PNAS...95.9226W.
- ↑ "Protein semisynthesis and expressed protein ligation: chasing a protein's tail". Curr Opin Chem Biol 9 (6): 561–9. 2005. doi:10.1016/j.cbpa.2005.09.018. PMID 16226484.
- ↑ "Protein ligation: an enabling technology for the biophysical analysis of proteins". Nat. Methods 3 (6): 429–38. 2006. doi:10.1038/nmeth886. PMID 16721376.
- ↑ de Grey, Aubrey D.N.J (2000). "Mitochondrial gene therapy: an arena for the biomedical use of inteins". Trends in Biotechnology 18 (9): 394–399. doi:10.1016/S0167-7799(00)01476-1. ISSN 0167-7799. PMID 10942964.
- ↑ 25.0 25.1 Chong, Shaorong; Mersha, Fana B; Comb, Donald G; Scott, Melissa E; Landry, David; Vence, Luis M; Perler, Francine B; Benner, Jack et al. (1997). "Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element". Gene 192 (2): 271–281. doi:10.1016/S0378-1119(97)00105-4. ISSN 0378-1119. PMID 9224900.
- ↑ 26.0 26.1 Fong, Baley A; Wood, David W (2010). "Expression and purification of ELP-intein-tagged target proteins in high cell density E. coli fermentation". Microbial Cell Factories 9 (1): 77. doi:10.1186/1475-2859-9-77. ISSN 1475-2859. PMID 20959011.
- ↑ Xing, Lei; Wu, Wei; Zhou, Bihong; Lin, Zhanglin (2011). "Streamlined protein expression and purification using cleavable self-aggregating tags". Microbial Cell Factories 10 (1): 42. doi:10.1186/1475-2859-10-42. ISSN 1475-2859. PMID 21631955.
- ↑ Shah, Neel H.; Muir, Tom W. (2013-12-24). "Inteins: nature's gift to protein chemists" (in en). Chemical Science 5 (2): 446–461. doi:10.1039/C3SC52951G. ISSN 2041-6539. PMID 24634716.
- ↑ Huet, Gaëlle; Castaing, Jean-Philippe; Fournier, Didier; Daffé, Mamadou; Saves, Isabelle (May 2006). "Protein Splicing of SufB Is Crucial for the Functionality of the Mycobacterium tuberculosis SUF Machinery" (in en). Journal of Bacteriology 188 (9): 3412–3414. doi:10.1128/JB.188.9.3412-3414.2006. ISSN 0021-9193. PMID 16621837.
- ↑ Chan, Hon; Pearson, C. Seth; Green, Cathleen M.; Li, Zhong; Zhang, Jing; Belfort, Georges; Shekhtman, Alex; Li, Hongmin et al. (October 2016). "Exploring Intein Inhibition by Platinum Compounds as an Antimicrobial Strategy". Journal of Biological Chemistry 291 (43): 22661–22670. doi:10.1074/jbc.m116.747824. ISSN 0021-9258. PMID 27609519.
- ↑ Li, Zhong; Tharappel, Anil Mathew; Xu, Jimin; Lang, Yuekun; Green, Cathleen M.; Zhang, Jing; Lin, Qishan; Chaturvedi, Sudha et al. (2021-01-12). "Small-molecule inhibitors for the Prp8 intein as antifungal agents" (in en). Proceedings of the National Academy of Sciences 118 (2): e2008815118. doi:10.1073/pnas.2008815118. ISSN 0027-8424. PMID 33397721. Bibcode: 2021PNAS..11808815L.
Further reading
- Gogarten, J Peter; Elena Hilario (2006). "Inteins, introns, and homing endonucleases: recent revelations about the life cycle of parasitic genetic elements". BMC Evol Biol 6 (1): 94. doi:10.1186/1471-2148-6-94. ISSN 1471-2148. PMID 17101053.
External links
- The Intein Database
- Shmuel Pietrokovski's Intein database
- Short review
- Starokadomskyy PL. Protein Splicing, 2007
- Protein splicing mechanism and intein structure
- Protein+Splicing at the US National Library of Medicine Medical Subject Headings (MeSH)
 |