Biology:Opisthorchis viverrini
| Opisthorchis viverrini | |
|---|---|
| |
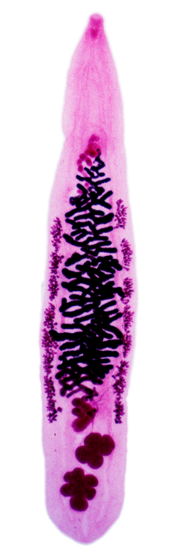
| An adult Opisthorchis viverrini showing (from top) oral sucker, pharynx, caecum, ventral sucker, vitellaria, uterus, ovary, Mehlis gland, testes, excretory bladder. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Trematoda |
| Order: | Plagiorchiida |
| Family: | Opisthorchiidae |
| Genus: | Opisthorchis |
| Species: | O. viverrini
|
| Binomial name | |
| Opisthorchis viverrini (Poirier, 1886) Stiles & Hassal, 1896
| |
| Synonyms[1] | |
| |
Opisthorchis viverrini, common name Southeast Asian liver fluke, is a food-borne trematode parasite from the family Opisthorchiidae that infects the bile duct. People are infected after eating raw or undercooked fish.[2] Infection with the parasite is called opisthorchiasis. O. viverrini infection also increases the risk of cholangiocarcinoma, a cancer of the bile ducts.[3]
A small, leaf-like fluke, O. viverrini completes its lifecycle in three different animals. Snails of the species Bithynia are the first intermediate hosts, fish belonging to the family Cyprinidae are the second intermediate host, and the definitive hosts are humans and other mammals such as dogs, cats, rats, and pigs. It was first discovered in the Indian fishing cat (Prionailurus viverrus) by M.J. Poirier in 1886. The first human case was discovered by Robert Thomson Leiper in 1915.
O. viverrini (together with Clonorchis sinensis and Opisthorchis felineus) is one of the three most medically important species in the family Opisthorchiidae.[4] In fact O. viverrini and C. sinensis are capable of causing cancer in humans, and are classified by the International Agency for Research on Cancer as a group 1 biological carcinogen in 2009.[5][6][7] O. viverrini is found in Thailand, the Laos, Vietnam, and Cambodia.[8] It is most widely distributed in northern Thailand, with high prevalence in humans, while central Thailand has a low rate of prevalence.[9][10]
Discovery
O. viverrini was first described by a French parasitologist Jules Poirier in 1886, who discovered the parasite in an Indian fishing cat (Prionailurus viverrus), originally from Southeast Asia, that died in the Zoological Gardens attached to the National Museum of Natural History in Paris. He named it Distomum viverrini.[11][12] American parasitologists Charles Wardell Stiles and Albert Hassall redescribed it and assigned it to the existing genus Opisthorchis (created by a French zoologist Raphaël Blanchard) in 1891. The first human specimen was described by a British parasitologist Robert Thomson Leiper in 1915, but without knowing the exact parasite. (He simply reported it as "Notes of the occurrence of parasites presumably rare in man.") Leiper received the specimens from an Irish medical doctor, Arthur Francis George Kerr, who had collected them from the post mortem examination of two prisoners at a jail in Chiang Mai, northern Thailand. In the next year, Kerr himself reported from investigation of 230 male prisoners that 39 (17 percent) of them had the infection. Kerr initially misidentified the parasite as O. felineus, an already known human parasite, because of their close resemblance.[13] C. Prommas also reported O. felineus in 1927 from an autopsy of a 17-year-old Thai male residing in Roi Et, northeast Thailand.[14] It was in 1955 when Elvio H. Sadun from the U. S. Public Health Service analysed the cases of opisthorchiasis in Thailand and concluded that all the infections were due to O. viverrini.[15] A systematic comparison in 1965 confirmed the differences from O. felineus.[16]
Description
Structurally, O. viverrini is basically similar to C. sinensis and O. felineus, but it is slightly smaller than the two flukes. The body of an adult O. viverrini is flat (dorsoventrally flattened) like a leaf, shaped like a lancet, and can be seen through (transparent). They are monoecious, so no male or female individuals exist; each fluke has the complete sets of both male and female reproductive systems. A typical individual is 7 mm long and 1.5 mm wide. The anterior end is more pointed and marked by a mouth-like structure called oral sucker. About 1.5 mm behind the oral sucker is a similar structure called ventral sucker. These suckers are the organs of attachment. Two testes are seen towards the posterior end. The testes are lobed in contrast to the branched (dendritic) testes of C.sinensis.[1] It is connected to the seminal vesicle, which is a coiled tube running up to the ejaculatory duct, which in turn opens through a small opening called genital pore just in front of the ventral sucker. Two ovaries are situated in front of the testes, and they form several lobes. The uterus runs along the ejaculatory duct and opens at the genital pore. A sac-like S-shaped tube called excretory bladder is between the two testes. The remaining body spaces are mostly occupied by a highly branched glandular organ called vitellaria (often called vitelline glands). Unlike the anterior end, the posterior end is rounded.[5][17]
The eggs of O. viverrini are 30 × 12 μm in size and they are slightly narrower and more regularly ovoid than in C. sinensis.[1] The eggs are visually indistinguishable in Kato technique smears from other eggs of flukes from other fluke family Heterophyidae.[18]
The infective larvae, metacercariae, of O. viverrini are brownish and elliptical, with two nearly equal-sized suckers – the oral sucker and the ventral sucker. They are 0.19–0.25 × 0.15–0.22 mm in size.[18]
Lifecycle
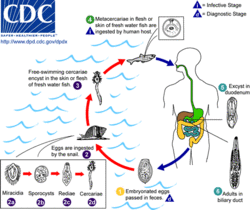
O. viverrini is a hermaphroditic liver fluke. Similar to C. sinensis and O. felineus, it requires three different hosts to complete its lifecycle. Freshwater snails are the first intermediate hosts in which asexual reproduction takes place, and freshwater fishes belonging to the family Cyprinidae) are second intermediate hosts in which larval development occurs. Fish–eating (piscivorous) mammals, including humans, dogs, and cats, act as definitive hosts, in which sexual reproduction occurs.[8] As a result of poor sanitation practices and inadequate sewerage infrastructure, O. viverrini-infected people pass the trematode's eggs in their feces into bodies of fresh water from where snails become infected.[2]
First intermediate host
The first intermediate hosts include freshwater snails of the genus Bithynia.[10] The only known host is Bithynia siamensis (that include all its three subspecies).[19] Snails are infected by the free-swimming larvae called miracidia in water bodies where faecal matters of infected mammals are deposited. Inside the snail tissue, the miracidia grow into sposocysts, that contain spore-like daughter cells. The daughter cells called rediae multiply and develop into numerous larvae called cercariae. Each cercaria has a large head and a long tail. The cercariae escape from the snail and enter the water body again as free-swimming larvae. Their tails act as a propeller for swimming and they actively search for a fish host.[12]
Second intermediate host
The cercaria then locates a cyprinoid fish, encysts in the fins, skin, and musculature of the fish, and becomes a metacercaria.[2] Habitats of second intermediate hosts of O. viverrini include freshwater habitats with stagnant or slow-moving waters (ponds, river, aquaculture, swamps, rice fields).[20]
In 1965, 9 fish hosts of O. viverrini were known.[16] Up to 2002, 15 species of fishes from seven genera of the family Cyprinidae were known to serve as second intermediate host.[1] Further research by Rim et al. (2008) showed an additional five host species. The known hosts include Puntius brevis, P. gonionotus, P. orphoides, P. proctozysron, P. viehoeveri, Hampala dispar, H. macrolepidota, Cyclocheilichthys armatus, C. repasson, Labiobarbus lineatus, Esomus metallicus, Mystacoleucus marginatus, Puntioplites falcifer, Onychostoma elongatum, Osteochilus hasseltii, Hypsibarbus lagleri, and Barbodes gonionotus.[18]
Definitive host

The metacercarial stage is infective to humans and other fish-eating mammals, including dogs, cats,[8] rats, and pigs.[22] Fish contain more metacercaria from September to February, before the dry season,[16] and this is when humans are usually infected.[1] Infection is acquired when people ingest raw or undercooked fish.[2] Dishes of raw fish are common in the cuisine of Laos and the cuisine of Thailand: koi pla, raw fish in spicy salad larb pla,[19] salted semifermented fish dishes called pla ra,[1] pla som[19] and som fak.[18] The natural definitive host is the leopard cat (Prionailurus bengalensis).[1] The young adult worm escapes from the metacercarial cyst in the upper small intestine and then migrates through the ampulla of Vater into the biliary tree, where it develops to sexual maturity over 4–6 weeks, thus completing the lifecycle.[2]
The adult worms primarily live in the bile duct, gall bladder, and sometimes in the pancreatic duct. Although they are hermaphrodites, reproduction is by cross fertilization (two individuals exchanging their gametes). Fertilized eggs are laid in the bile duct and are discharged along the bile juice into the intestine, and finally released in the environment along with the faeces.[17] An individual fluke may shed as many as 200 eggs in a day.[2] The exact lifespan is not known, but is estimated to be more than 25 years.[5]
Prevalence
O. viverrini remains a major public health problem in the Mekong Basin in Southeast Asia. It is endemic in Thailand, the Lao People's Democratic Republic, Vietnam, and Cambodia.[23][24][25][10] It is most prevalent in Thailand, and for this reason Thailand has the highest incidence of opisthorchiasis-associated cancer, cholangiocarcinoma (CCA) in the world. About 9.6% of the total population of Thailand is estimated to be infected. It is most abundant in northern Thailand, while it occurs moderately in central Thailand. According to the five-year national survey from 2010 to 2015, the highest incidence reached up to 45.7% of the population in northern Thailand.[26] However, there is no record of opisthorchiasis due to O. viverrini in southern Thailand.[9] School children are most infected, and the infection was very high before 1984, after which there was mass treatment programme, and the prevalence sharply declined after 1994.[27] A national survey in Lao PDR (under the project of Korea-Laos Collaborative Project for Control of Foodborne Trematode Infections in Lao PDR) between 2007 and 2011 indicates that it is the most prevalent helminth infection, amounting to 55.6% of the infection.[28] It is not highly prevalent in Vietnam, but accurate survey is difficult because it is often co-infected with other flukes such as Haplorchis pumilio, H. taichui, and C. sinensis. It is most abundant in the northern provinces.[29] It is least prevalent in Cambodia. A national survey between 2006 and 2011 showed that it is the second most prevalent helminth accounting for 5.7% of the total infection, after hookworm with 9.6% of the infection.[30]
Effect on human health
Generally, opisthorchiasis due to O. viverrini is harmless without any clinical symptoms. Mild symptoms may appear such as dyspepsia, abdominal pain, constipation, or diarrhoea. However, under severe infection, enlargement of liver (hepatomegaly) and malnutrition are observed. In rare cases, cholangitis, cholecystitis, and cholangiocarcinoma can also develop. In humans, O. viverrini inhabits mainly the bile ducts, and rarely, the gall bladder and pancreatic duct. Heavy infection can produce problems in the liver, gall bladder, and bile ducts. The bile ducts of heavily infected patients are usually dilated and indicate fibrosis.[31][32] Pathological effects on the bile ducts include inflammation, epithelial desquamation, goblet-cell metaplasia, epithelial and adenomatous hyperplasia, and periductal fibrosis.[33] The collective effects in addition to specific parasite secretion and the host's immune reactions account for the development of cholangiocarcinoma. The infection is not immediately life-threatening; cancer develops after 30–40 years, but death occurs very fast, within 3–6 months of diagnosis.[21]
Medical care and loss of wages caused by O. viverrini in Laos and in Thailand costs about US$120 million annually,[1] primarily in northeast Thailand.[4]
Infections with O. viverrini and of other liver flukes in Asia affect the poor and poorest people.[34] Opisthorchiasis has received less attention in comparison to other diseases, and it is a neglected disease in Asia.[34] There is no approved drug for the infection; however, Swiss researchers have tested tribendimidine and achieved a 70% cure rate.[35] Surgery and supportive treatment are complicated and generally unavailable in the endemic areas.[36] A general trematocide praziquantel is used for the infection, but is not technically recommended.[37] In addition to praziquantel other commonly used anthelmintics such as albendazole, artesunate, and miltefosine are found to be effective on the cercariae but not on the metacercariae.[38] Its ability to cause cancer is worsened by the discovery that its infection is often associated with those of Helicobacter species (including H. pylori, which is primarily associated with ulcers, but also may cause stomach cancers).[39][40]
Genetics
O. viverrini has 12 (six pairs) of chromosomes, i.e. 2n = 12.[2] The draft genome and transcriptomes were published in 2014. Its genome is 634.5 MB in size. The species has 16,379 protein-coding genes.[41]
See also
- The Integrated Opisthorchiasis Control Program
References
This article incorporates CC-BY-2.5 text from references[8][21][42] and CC-BY-2.0 text from the reference.[2]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Muller, Ralph; Wakelin, Derek (2002). Worms and Human Disease (2 ed.). Wallingford [UK]: CABI. pp. 43–44. ISBN 978-0-85-199516-8. https://books.google.com/books?id=bWtCMIGF_FMC.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini". BMC Genomics 8: 189. June 2007. doi:10.1186/1471-2164-8-189. PMID 17587442..
- ↑ "Opisthorchiasis and cholangiocarcinoma in Southeast Asia: an unresolved problem". International Journal of General Medicine 10: 227–237. 2017. doi:10.2147/IJGM.S133292. PMID 28848361.
- ↑ 4.0 4.1 "Trematodes of the family Opisthorchiidae: a minireview". The Korean Journal of Parasitology 39 (3): 209–21. September 2001. doi:10.3347/kjp.2001.39.3.209. PMID 11590910.
- ↑ 5.0 5.1 5.2 "Opisthorchis viverrini: the carcinogenic human liver fluke". World Journal of Gastroenterology 14 (5): 666–74. February 2008. doi:10.3748/wjg.14.666. PMID 18205254.
- ↑ "The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer". Trends in Parasitology 28 (10): 395–407. October 2012. doi:10.1016/j.pt.2012.07.006. PMID 22947297.
- ↑ American Cancer Society (2013). "Known and Probable Human Carcinogens". cancer.org. American Cancer Society, Inc.. http://www.cancer.org/cancer/cancercauses/othercarcinogens/generalinformationaboutcarcinogens/known-and-probable-human-carcinogens.
- ↑ 8.0 8.1 8.2 8.3 Jones, Malcolm K, ed (June 2010). "Unlocking the transcriptomes of two carcinogenic parasites, Clonorchis sinensis and Opisthorchis viverrini". PLOS Neglected Tropical Diseases 4 (6): e719. doi:10.1371/journal.pntd.0000719. PMID 20582164.
- ↑ 9.0 9.1 "Opisthorchiasis control in Thailand". Acta Tropica 88 (3): 229–32. November 2003. doi:10.1016/j.actatropica.2003.01.002. PMID 14611877.
- ↑ 10.0 10.1 10.2 Rachprakhon, Phuphitchan; Purivirojkul, Watchariya (2021). "Very low prevalence of Opisthorchis viverrini s.l. cercariae in Bithynia siamensis siamensis snails from the canal network system in the Bangkok Metropolitan Region, Thailand". Parasite 28: 2. doi:10.1051/parasite/2020072. ISSN 1776-1042. PMID 33416490.

- ↑ Poirier, J. (1886). "Distomum viverrini" (in fr). Bulletin de la Société philomathique de Paris 6: 27–29. https://archive.org/stream/bulletindelasoci910corb/bulletindelasoci910corb_djvu.txt.
- ↑ 12.0 12.1 Saijuntha, W.; Sithithaworn, P.; Kaitsopit, N.; Andrews, R.H.; Petney, T.N (2014). "Liver Flukes: Clonorchis and Opisthorchis". in Toledo, Rafael; Fried, Bernard. Digenetic Trematodes. New York (US): Springer. pp. 153–200. ISBN 978-1-49-390914-8. https://books.google.com/books?id=PPW_AwAAQBAJ.
- ↑ "Discovery of human opisthorchiasis: a mysterious history". Parasitology International 61 (1): 3–4. March 2012. doi:10.1016/j.parint.2011.08.012. PMID 21867771.
- ↑ Prommas, C. (1927). "Report of a Case of Opisthorchis Felineus in Siam". Annals of Tropical Medicine & Parasitology 21 (1): 9–10. doi:10.1080/00034983.1927.11684513.
- ↑ "Studies on Opisthorchis viverrini in Thailand". American Journal of Hygiene 62 (2): 81–115. September 1955. doi:10.1093/oxfordjournals.aje.a119772. PMID 13258561.
- ↑ 16.0 16.1 16.2 "Opisthorchis Viverrini in Thailand--The Life Cycle and Comparison with O. Felineus". The Journal of Parasitology 51 (2): 207–14. April 1965. doi:10.2307/3276083. PMID 14275209., JSTOR.
- ↑ 17.0 17.1 "Opisthorchis viverrini and Opisthorchis felineus". Encyclopedia of Food Safety (First ed.). San Diego (US): Academic Press. 2014. pp. 170–178. ISBN 978-0-12-378613-5. https://books.google.com/books?id=mX1XAQAAQBAJ&pg=RA1-PA171.
- ↑ 18.0 18.1 18.2 18.3 "Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR". The Korean Journal of Parasitology 46 (4): 253–60. December 2008. doi:10.3347/kjp.2008.46.4.253. PMID 19127332.
- ↑ 19.0 19.1 19.2 World Health Organization (1995). Control of Foodborne Trematode Infection. WHO Technical Report Series. 849. PDF part 1, PDF part 2. page 89-91.
- ↑ "Artemisinins and synthetic trioxolanes in the treatment of helminth infections". Current Opinion in Infectious Diseases 20 (6): 605–12. December 2007. doi:10.1097/QCO.0b013e3282f19ec4. PMID 17975411.
- ↑ 21.0 21.1 21.2 "Liver fluke induces cholangiocarcinoma". PLOS Medicine 4 (7): e201. July 2007. doi:10.1371/journal.pmed.0040201. PMID 17622191..
- ↑ "Fish-borne parasitic zoonoses: status and issues". International Journal for Parasitology 35 (11–12): 1233–54. October 2005. doi:10.1016/j.ijpara.2005.07.013. PMID 16143336.
- ↑ "Opisthorchis viverrini infections and associated risk factors in a lowland area of Binh Dinh Province, Central Vietnam". Acta Tropica 157: 151–7. May 2016. doi:10.1016/j.actatropica.2016.01.029. PMID 26872984.
- ↑ "Prevalence of Opisthorchis viverrini infection in humans and fish in Kratie Province, Cambodia". Acta Tropica 124 (3): 215–20. December 2012. doi:10.1016/j.actatropica.2012.08.011. PMID 22935318.
- ↑ "Field survey focused on Opisthorchis viverrini infection in five provinces of Cambodia". Parasitology International 63 (2): 366–73. April 2014. doi:10.1016/j.parint.2013.12.003. PMID 24342554. http://www.lib.kobe-u.ac.jp/repository/thesis2/d2/D2003261.pdf.
- ↑ "Review and Current Status of Opisthorchis viverrini Infection at the Community Level in Thailand". Asian Pacific Journal of Cancer Prevention 16 (16): 6825–30. 2015. doi:10.7314/apjcp.2015.16.16.6825. PMID 26514452.
- ↑ "Changing patterns of prevalence in Opisthorchis viverrini sensu lato infection in children and adolescents in northeast Thailand". Acta Tropica 164: 469–472. December 2016. doi:10.1016/j.actatropica.2016.10.017. PMID 27794488.
- ↑ "Prevalence of helminthic infections among inhabitants of Lao PDR". The Korean Journal of Parasitology 52 (1): 51–6. February 2014. doi:10.3347/kjp.2014.52.1.51. PMID 24623882.
- ↑ "Clonorchis sinensis and Opisthorchis spp. in Vietnam: current status and prospects". Transactions of the Royal Society of Tropical Medicine and Hygiene 110 (1): 13–20. January 2016. doi:10.1093/trstmh/trv103. PMID 26740358.
- ↑ "Prevalence of intestinal helminths among inhabitants of Cambodia (2006–2011)". The Korean Journal of Parasitology 52 (6): 661–6. December 2014. doi:10.3347/kjp.2014.52.6.661. PMID 25548418.
- ↑ "Peritoneoscopic findings in 203 patients with Opisthorchis viverrini infection". Gastrointestinal Endoscopy 33 (1): 18–20. February 1987. doi:10.1016/S0016-5107(87)71478-3. PMID 2951293.
- ↑ "Multistage carcinogenesis of liver-fluke-associated cholangiocarcinoma in Thailand". Princess Takamatsu Symposia 22: 77–86. 1991. PMID 1668894.
- ↑ "Pathobiology of opisthorchiasis: an update". Acta Tropica 88 (3): 209–20. November 2003. doi:10.1016/j.actatropica.2003.08.002. PMID 14611875.
- ↑ 34.0 34.1 Loukas, Alex, ed (May 2008). "Concerted action is needed to tackle liver fluke infections in Asia". PLOS Neglected Tropical Diseases 2 (5): e232. doi:10.1371/journal.pntd.0000232. PMID 18509525..
- ↑ "New drug shows promise against Asian liver fluke". 2010-11-24. https://www.reuters.com/article/us-liver-fluke-drugs-idUSTRE6AO00520101125.
- ↑ "Opisthorchis viverrini: an underestimated parasite in world health". Trends in Parasitology 24 (11): 497–501. November 2008. doi:10.1016/j.pt.2008.08.011. PMID 18930439.
- ↑ "Infection with Opisthorchis viverrini and use of praziquantel among a working-age population in northeast Thailand". Asian Pacific Journal of Cancer Prevention 14 (5): 2963–6. 2013. doi:10.7314/apjcp.2013.14.5.2963. PMID 23803062.
- ↑ "Effects of albendazole, artesunate, praziquantel and miltefosine, on Opisthorchis viverrini cercariae and mature metacercariae". Asian Pacific Journal of Tropical Medicine 10 (2): 126–133. February 2017. doi:10.1016/j.apjtm.2017.01.019. PMID 28237476.
- ↑ "Association between Helicobacter spp. infections and hepatobiliary malignancies: a review". World Journal of Gastroenterology 21 (5): 1414–23. February 2015. doi:10.3748/wjg.v21.i5.1414. PMID 25663761.
- ↑ "Helicobacter Species are Possible Risk Factors of Cholangiocarcinoma". Asian Pacific Journal of Cancer Prevention 17 (1): 37–44. 2016. doi:10.7314/apjcp.2016.17.1.37. PMID 26838240.
- ↑ "The Opisthorchis viverrini genome provides insights into life in the bile duct". Nature Communications 5: 4378. July 2014. doi:10.1038/ncomms5378. PMID 25007141. Bibcode: 2014NatCo...5.4378Y.
- ↑ Sripa, Banchob, ed (2009). "A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand". PLOS Neglected Tropical Diseases 3 (1): e367. doi:10.1371/journal.pntd.0000367. PMID 19156191..
Further reading
- "Opisthorchis viverrini and opisthorchiasis: a historical review and future perspective". Acta Tropica 88 (3): 171–6. November 2003. doi:10.1016/j.actatropica.2003.01.001. PMID 14611871.
- "Taxonomy and biology of liver flukes". Acta Tropica 88 (3): 177–86. November 2003. doi:10.1016/j.actatropica.2003.05.001. PMID 14611872.
- "Morphology and ultrastructure of the redia and pre-emergent cercaria of Opisthorchis viverrini (Trematoda: Digenea) in the intermediate host Bithynia siamensis goniomphalus (Prosobranchia: Bithyniidae)". Applied Parasitology 36 (2): 136–54. May 1995. PMID 7550441.
- "The ultrastructure of helminth. VI. The body wall of Opisthorchis viverrini (Poirier, 1886)". Acta Medicinae Okayama 25 (2): 129–42. 1971. PMID 4333630..
External links
- Public Health Agency of Canada Opisthorchis spp. – Pathogen Safety Data Sheet
- Genome info at WormBase
- Info at Encyclopedia of Life
- Taxonomy at UniProt
- DPDx Laboratory Identification of Parasites of Public Health Concern(CDC)
- The Program in Human Biology, Stanford University
Wikidata ☰ Q135523 entry
 |