Medicine:Cholangiocarcinoma
| Cholangiocarcinoma | |
|---|---|
| Other names | Bile duct cancer, cancer of the bile duct[1] |
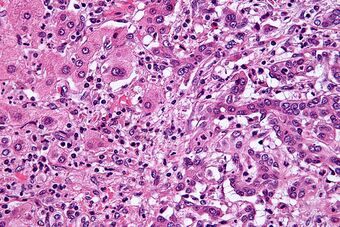 | |
| Micrograph of an intrahepatic cholangiocarcinoma (right of image) adjacent to normal liver cells (left of image). H&E stain. | |
| Pronunciation | |
| Specialty | Oncology |
| Symptoms | Abdominal pain, yellowish skin, weight loss, generalized itching, fever[1] |
| Usual onset | 70 years old[3] |
| Types | Intrahepatic, perihilar, distal[3] |
| Risk factors | Primary sclerosing cholangitis, ulcerative colitis, infection with certain liver flukes, some congenital liver malformations[1] |
| Diagnostic method | Confirmed by examination of the tumor under a microscope[4] |
| Treatment | Surgical resection, chemotherapy, radiation therapy, stenting procedures, liver transplantation[1] |
| Prognosis | Generally poor[5] |
| Frequency | 1–2 people per 100,000 per year (Western world)[6] |
Cholangiocarcinoma, also known as bile duct cancer, is a type of cancer that forms in the bile ducts.[2] Symptoms of cholangiocarcinoma may include abdominal pain, yellowish skin, weight loss, generalized itching, and fever.[1] Light colored stool or dark urine may also occur.[4] Other biliary tract cancers include gallbladder cancer and cancer of the ampulla of Vater.[7]
Risk factors for cholangiocarcinoma include primary sclerosing cholangitis (an inflammatory disease of the bile ducts), ulcerative colitis, cirrhosis, hepatitis C, hepatitis B, infection with certain liver flukes, and some congenital liver malformations.[1][3][8] However, most people have no identifiable risk factors.[3] The diagnosis is suspected based on a combination of blood tests, medical imaging, endoscopy, and sometimes surgical exploration.[4] The disease is confirmed by examination of cells from the tumor under a microscope.[4] It is typically an adenocarcinoma (a cancer that forms glands or secretes mucin).[3]
Cholangiocarcinoma is typically incurable at diagnosis which is why early detection is ideal.[9][1] In these cases palliative treatments may include surgical resection, chemotherapy, radiation therapy, and stenting procedures.[1] In about a third of cases involving the common bile duct and less commonly with other locations the tumor can be completely removed by surgery offering a chance of a cure.[1] Even when surgical removal is successful chemotherapy and radiation therapy are generally recommended.[1] In certain cases surgery may include a liver transplantation.[3] Even when surgery is successful the 5-year survival is typically less than 50%.[6]
Cholangiocarcinoma is rare in the Western world, with estimates of it occurring in 0.5–2 people per 100,000 per year.[1][6] Rates are higher in Southeast Asia where liver flukes are common.[5] Rates in parts of Thailand are 60 per 100,000 per year.[5] It typically occurs in people in their 70s; however, in those with primary sclerosing cholangitis it often occurs in the 40s.[3] Rates of cholangiocarcinoma within the liver in the Western world have increased.[6]
Signs and symptoms
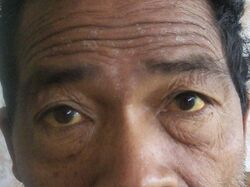
The most common physical indications of cholangiocarcinoma are abnormal liver function tests, jaundice (yellowing of the eyes and skin occurring when bile ducts are blocked by tumor), abdominal pain (30–50%), generalized itching (66%), weight loss (30–50%), fever (up to 20%), and changes in the color of stool or urine.[10] To some extent, the symptoms depend upon the location of the tumor: people with cholangiocarcinoma in the extrahepatic bile ducts (outside the liver) are more likely to have jaundice, while those with tumors of the bile ducts within the liver more often have pain without jaundice.[11]
Blood tests of liver function in people with cholangiocarcinoma often reveal a so-called "obstructive picture", with elevated bilirubin, alkaline phosphatase, and gamma glutamyl transferase levels, and relatively normal transaminase levels. Such laboratory findings suggest obstruction of the bile ducts, rather than inflammation or infection of the liver parenchyma, as the primary cause of the jaundice.[12]
Risk factors
Although most people present without any known risk factors evident, a number of risk factors for the development of cholangiocarcinoma have been described. In the Western world, the most common of these is primary sclerosing cholangitis (PSC), an inflammatory disease of the bile ducts which is closely associated with ulcerative colitis (UC).[13] Epidemiologic studies have suggested that the lifetime risk of developing cholangiocarcinoma for a person with PSC is on the order of 10–15%,[14] although autopsy series have found rates as high as 30% in this population.[15] For inflammatory bowel disease patients with altered DNA repair functions, the progression from PSC to cholangiocarcinoma may be a consequence of DNA damage resulting from biliary inflammation and bile acids.[16]
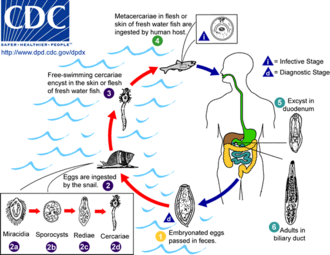
Certain parasitic liver diseases may be risk factors as well. Colonization with the liver flukes Opisthorchis viverrini (found in Thailand, Laos PDR, and Vietnam)[17][18][19] or Clonorchis sinensis (found in China, Taiwan, eastern Russia, Korea, and Vietnam)[20][21] has been associated with the development of cholangiocarcinoma. Control programs (Integrated Opisthorchiasis Control Program) aimed at discouraging the consumption of raw and undercooked food have been successful at reducing the incidence of cholangiocarcinoma in some countries.[22] People with chronic liver disease, whether in the form of viral hepatitis (e.g. hepatitis B or hepatitis C),[23][24][25] alcoholic liver disease, or cirrhosis of the liver due to other causes, are at significantly increased risk of cholangiocarcinoma.[26][27] HIV infection was also identified in one study as a potential risk factor for cholangiocarcinoma, although it was unclear whether HIV itself or other correlated and confounding factors (e.g. hepatitis C infection) were responsible for the association.[26]
Infection with the bacteria Helicobacter bilis and Helicobacter hepaticus species can cause biliary cancer.[28]
Congenital liver abnormalities, such as Caroli disease (a specific type of five recognized choledochal cysts), have been associated with an approximately 15% lifetime risk of developing cholangiocarcinoma.[29][30] The rare inherited disorders Lynch syndrome II and biliary papillomatosis have also been found to be associated with cholangiocarcinoma.[31][32] The presence of gallstones (cholelithiasis) is not clearly associated with cholangiocarcinoma. However, intrahepatic stones (called hepatolithiasis), which are rare in the West but common in parts of Asia, have been strongly associated with cholangiocarcinoma.[33][34][35] Exposure to Thorotrast, a form of thorium dioxide which was used as a radiologic contrast medium, has been linked to the development of cholangiocarcinoma as late as 30–40 years after exposure; Thorotrast was banned in the United States in the 1950s due to its carcinogenicity.[36][37][38]
Pathophysiology
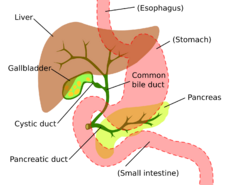
Cholangiocarcinoma can affect any area of the bile ducts, either within or outside the liver. Tumors occurring in the bile ducts within the liver are referred to as intrahepatic, those occurring in the ducts outside the liver are extrahepatic, and tumors occurring at the site where the bile ducts exit the liver may be referred to as perihilar. A cholangiocarcinoma occurring at the junction where the left and right hepatic ducts meet to form the common hepatic duct may be referred to eponymously as a Klatskin tumor.[39]
Although cholangiocarcinoma is known to have the histological and molecular features of an adenocarcinoma of epithelial cells lining the biliary tract, the actual cell of origin is unknown. Recent evidence has suggested that the initial transformed cell that generates the primary tumor may arise from a pluripotent hepatic stem cell.[40][41][42] Cholangiocarcinoma is thought to develop through a series of stages – from early hyperplasia and metaplasia, through dysplasia, to the development of frank carcinoma – in a process similar to that seen in the development of colon cancer.[43] Chronic inflammation and obstruction of the bile ducts, and the resulting impaired bile flow, are thought to play a role in this progression.[43][44][45]
Histologically, cholangiocarcinomas may vary from undifferentiated to well-differentiated. They are often surrounded by a brisk fibrotic or desmoplastic tissue response; in the presence of extensive fibrosis, it can be difficult to distinguish well-differentiated cholangiocarcinoma from normal reactive epithelium. There is no entirely specific immunohistochemical stain that can distinguish malignant from benign biliary ductal tissue, although staining for cytokeratins, carcinoembryonic antigen, and mucins may aid in diagnosis.[46] Most tumors (>90%) are adenocarcinomas.[47]
Diagnosis
Blood tests
There are no specific blood tests that can diagnose cholangiocarcinoma by themselves. Serum levels of carcinoembryonic antigen (CEA) and CA19-9 are often elevated, but are not sensitive or specific enough to be used as a general screening tool. However, they may be useful in conjunction with imaging methods in supporting a suspected diagnosis of cholangiocarcinoma.[48]
Abdominal imaging
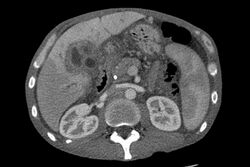
Ultrasound of the liver and biliary tree is often used as the initial imaging modality in people with suspected obstructive jaundice.[49][50] Ultrasound can identify obstruction and ductal dilatation and, in some cases, may be sufficient to diagnose cholangiocarcinoma.[51] Computed tomography (CT) scanning may also play an important role in the diagnosis of cholangiocarcinoma.[52][53][54]
Imaging of the biliary tree
While abdominal imaging can be useful in the diagnosis of cholangiocarcinoma, direct imaging of the bile ducts is often necessary. Endoscopic retrograde cholangiopancreatography (ERCP), an endoscopic procedure performed by a gastroenterologist or specially trained surgeon, has been widely used for this purpose. Although ERCP is an invasive procedure with attendant risks, its advantages include the ability to obtain biopsies and to place stents or perform other interventions to relieve biliary obstruction.[12] Endoscopic ultrasound can also be performed at the time of ERCP and may increase the accuracy of the biopsy and yield information on lymph node invasion and operability.[55] As an alternative to ERCP, percutaneous transhepatic cholangiography (PTC) may be utilized. Magnetic resonance cholangiopancreatography (MRCP) is a non-invasive alternative to ERCP.[56][57][58] Some authors have suggested that MRCP should supplant ERCP in the diagnosis of biliary cancers, as it may more accurately define the tumor and avoids the risks of ERCP.[59][60][61]
Surgery
Surgical exploration may be necessary to obtain a suitable biopsy and to accurately stage a person with cholangiocarcinoma. Laparoscopy can be used for staging purposes and may avoid the need for a more invasive surgical procedure, such as laparotomy, in some people.[62][63]
Pathology

Histologically, cholangiocarcinomas are classically well to moderately differentiated adenocarcinomas. Immunohistochemistry is useful in the diagnosis and may be used to help differentiate a cholangiocarcinoma from hepatocellular carcinoma and metastasis of other gastrointestinal tumors.[65] Cytological scrapings are often nondiagnostic,[66] as these tumors typically have a desmoplastic stroma and, therefore, do not release diagnostic tumor cells with scrapings.
Staging
Although there are at least three staging systems for cholangiocarcinoma (e.g. those of Bismuth, Blumgart, and the American Joint Committee on Cancer), none have been shown to be useful in predicting survival.[67] The most important staging issue is whether the tumor can be surgically removed, or whether it is too advanced for surgical treatment to be successful. Often, this determination can only be made at the time of surgery.[12]
General guidelines for operability include:[68][69]
- Absence of lymph node or liver metastases
- Absence of involvement of the portal vein
- Absence of direct invasion of adjacent organs
- Absence of widespread metastatic disease
Treatment
Cholangiocarcinoma is considered to be an incurable and rapidly lethal disease unless all the tumors can be fully resected (cut out surgically). Since the operability of the tumor can only be assessed during surgery in most cases,[70] a majority of people undergo exploratory surgery unless there is already a clear indication that the tumor is inoperable.[12] However, the Mayo Clinic has reported significant success treating early bile duct cancer with liver transplantation using a protocolized approach and strict selection criteria.[71]
Adjuvant therapy followed by liver transplantation may have a role in treatment of certain unresectable cases.[72] Locoregional therapies including transarterial chemoembolization (TACE), transarterial radioembolization (TARE) and ablation therapies have a role in intrahepatic variants of cholangiocarcinoma to provide palliation or potential cure in people who are not surgical candidates.[73]
Adjuvant chemotherapy and radiation therapy
If the tumor can be removed surgically, people may receive adjuvant chemotherapy or radiation therapy after the operation to improve the chances of cure. If the tissue margins are negative (i.e. the tumor has been totally excised), adjuvant therapy is of uncertain benefit. Both positive[74][75] and negative[11][76][77] results have been reported with adjuvant radiation therapy in this setting, and no prospective randomized controlled trials have been conducted as of March 2007. Adjuvant chemotherapy appears to be ineffective in people with completely resected tumors.[78][79] The role of combined chemoradiotherapy in this setting is unclear. However, if the tumor tissue margins are positive, indicating that the tumor was not completely removed via surgery, then adjuvant therapy with radiation and possibly chemotherapy is generally recommended based on the available data.[80] [81]
Treatment of advanced disease
The majority of cases of cholangiocarcinoma present as inoperable (unresectable) disease[82] in which case people are generally treated with palliative chemotherapy, with or without radiotherapy. Chemotherapy has been shown in a randomized controlled trial to improve quality of life and extend survival in people with inoperable cholangiocarcinoma.[83] There is no single chemotherapy regimen which is universally used, and enrollment in clinical trials is often recommended when possible.[81] Chemotherapy agents used to treat cholangiocarcinoma include 5-fluorouracil with leucovorin,[84] gemcitabine as a single agent,[85] or gemcitabine plus cisplatin,[86] irinotecan,[87] or capecitabine.[88] A small pilot study suggested possible benefit from the tyrosine kinase inhibitor erlotinib in people with advanced cholangiocarcinoma.[89] Radiation therapy appears to prolong survival in people with resected extrahepatic cholangiocarcinoma,[90] and the few reports of its use in unresectable cholangiocarcinoma appear to show improved survival, but numbers are small.[6]
Infigratinib (Truseltiq) is a tyrosine kinase inhibitor of fibroblast growth factor receptor (FGFR) that was approved for medical use in the United States in May 2021.[91] It is indicated for the treatment of people with previously treated locally advanced or metastatic cholangiocarcinoma harboring an FGFR2 fusion or rearrangement.[91]
Pemigatinib (Pemazyre) is a kinase inhibitor of fibroblast growth factor receptor 2 (FGFR2) that was approved for medical use in the United States in April 2020.[92] It is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test.
Ivodesinib (Tibsovo) is a small molecule inhibitor of isocitrate dehydrogenase 1. The FDA approved ivosidenib in August 2021 for adults with previously treated, locally advanced or metastatic cholangiocarcinoma with an isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.[93]
Durvalumab, (Imfinzi) is an immune checkpoint inhibitor that blocks the PD-L1 protein on the surface of immune cells, thereby allowing the immune system to recognize and attack tumor cells. In Phase III clinical trials, durvalumab, in combination with standard-of-care chemotherapy, demonstrated a statistically significant and clinically meaningful improvement in overall survival and progression-free survival versus chemotherapy alone as a 1st-line treatment for patients with advanced biliary tract cancer.[94]
Futibatinib (Lytgobi) was approved for medical use in the United States in September 2022.[95]
Prognosis
Surgical resection offers the only potential chance of cure in cholangiocarcinoma. For non-resectable cases, the five-year survival rate is 0% where the disease is inoperable because distal lymph nodes show metastases,[96] and less than 5% in general.[97] Overall mean duration of survival is less than 6 months in people with metastatic disease.[98]
For surgical cases, the odds of cure vary depending on the tumor location and whether the tumor can be completely, or only partially, removed. Distal cholangiocarcinomas (those arising from the common bile duct) are generally treated surgically with a Whipple procedure; long-term survival rates range from 15 to 25%, although one series reported a five-year survival of 54% for people with no involvement of the lymph nodes.[99] Intrahepatic cholangiocarcinomas (those arising from the bile ducts within the liver) are usually treated with partial hepatectomy. Various series have reported survival estimates after surgery ranging from 22 to 66%; the outcome may depend on involvement of lymph nodes and completeness of the surgery.[100] Perihilar cholangiocarcinomas (those occurring near where the bile ducts exit the liver) are least likely to be operable. When surgery is possible, they are generally treated with an aggressive approach often including removal of the gallbladder and potentially part of the liver. In patients with operable perihilar tumors, reported 5-year survival rates range from 20 to 50%.[101]
The prognosis may be worse for people with primary sclerosing cholangitis who develop cholangiocarcinoma, likely because the cancer is not detected until it is advanced.[15][102] Some evidence suggests that outcomes may be improving with more aggressive surgical approaches and adjuvant therapy.[103]
Epidemiology
| Country | IC (men/women) | EC (men/women) |
|---|---|---|
| U.S.A. | 0.60/0.43 | 0.70/0.87 |
| Japan | 0.23/0.10 | 5.87/5.20 |
| Australia | 0.70/0.53 | 0.90/1.23 |
| England/Wales | 0.83/0.63 | 0.43/0.60 |
| Scotland | 1.17/1.00 | 0.60/0.73 |
| France | 0.27/0.20 | 1.20/1.37 |
| Italy | 0.13/0.13 | 2.10/2.60 |
Cholangiocarcinoma is a relatively rare form of cancer; each year, approximately 2,000 to 3,000 new cases are diagnosed in the United States, translating into an annual incidence of 1–2 cases per 100,000 people.[105] Autopsy series have reported a prevalence of 0.01% to 0.46%.[82][106] There is a higher prevalence of cholangiocarcinoma in Asia, which has been attributed to endemic chronic parasitic infestation. The incidence of cholangiocarcinoma increases with age, and the disease is slightly more common in men than in women (possibly due to the higher rate of primary sclerosing cholangitis, a major risk factor, in men).[47] The prevalence of cholangiocarcinoma in people with primary sclerosing cholangitis may be as high as 30%, based on autopsy studies.[15]
Multiple studies have documented a steady increase in the incidence of intrahepatic cholangiocarcinoma; increases have been seen in North America, Europe, Asia, and Australia.[107] The reasons for the increasing occurrence of cholangiocarcinoma are unclear; improved diagnostic methods may be partially responsible, but the prevalence of potential risk factors for cholangiocarcinoma, such as HIV infection, has also been increasing during this time frame.[26]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Bile Duct Cancer (Cholangiocarcinoma) Treatment". 23 September 2020. https://www.cancer.gov/types/liver/hp/bile-duct-treatment-pdq#section/all.
- ↑ 2.0 2.1 "cholangiocarcinoma". 2 February 2011. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cholangiocarcinoma.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Cholangiocarcinoma". Lancet 383 (9935): 2168–79. June 2014. doi:10.1016/S0140-6736(13)61903-0. PMID 24581682.
- ↑ 4.0 4.1 4.2 4.3 "Bile Duct Cancer (Cholangiocarcinoma)". 5 July 2018. https://www.cancer.gov/types/liver/patient/about-bile-duct-cancer-pdq#section/all.
- ↑ 5.0 5.1 5.2 Bosman, Frank T. (2014). "Chapter 5.6: Liver cancer". in Stewart, Bernard W.; Wild, Christopher P. World Cancer Report. the International Agency for Research on Cancer, World Health Organization. pp. 403–12. ISBN 978-92-832-0443-5. https://publications.iarc.fr/_publications/media/download/5839/bc44643f904185d5c8eddb933480b5bc18b21dba.pdf.

- ↑ 6.0 6.1 6.2 6.3 6.4 "Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling". American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology. Annual Meeting 35 (36): e194-203. 2016. doi:10.1200/EDBK_160831. PMID 27249723.
- ↑ "Biliary tract cancers: SEOM clinical guidelines". Clinical & Translational Oncology 17 (12): 982–7. December 2015. doi:10.1007/s12094-015-1436-2. PMID 26607930.
- ↑ "Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis". Infectious Diseases of Poverty 7 (1): 44. May 2018. doi:10.1186/s40249-018-0434-3. PMID 29769113.
- ↑ Zhang, Tan; Zhang, Sina; Jin, Chen et al. (2021). "A Predictive Model Based on the Gut Microbiota Improves the Diagnostic Effect in Patients with Cholangiocarcinoma". Frontiers in Cellular and Infection Microbiology 11: 751795. doi:10.3389/fcimb.2021.751795. PMID 34888258.
- ↑ "Outcomes after curative resections of cholangiocarcinoma". Archives of Surgery 128 (8): 871–7; discussion 877–9. August 1993. doi:10.1001/archsurg.1993.01420200045008. PMID 8393652.
- ↑ 11.0 11.1 "Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors". Annals of Surgery 224 (4): 463–73; discussion 473–5. October 1996. doi:10.1097/00000658-199610000-00005. PMID 8857851.
- ↑ 12.0 12.1 12.2 12.3 Sleisenger and Fordtran's Gastrointestinal and Liver Disease (8th ed.). Saunders. 21 July 2006. pp. 1493–6. ISBN 978-1-4160-0245-1. https://archive.org/details/sleisengerfordtr0000unse_j1o5/page/1493.
- ↑ "Risk factors for biliary tract carcinogenesis". Annals of Oncology 10 (Suppl 4): 308–11. 1999. doi:10.1023/A:1008313809752. PMID 10436847.
- ↑ Epidemiologic studies which have addressed the incidence of cholangiocarcinoma in people with primary sclerosing cholangitis include the following:
- "Hepatic and extrahepatic malignancies in primary sclerosing cholangitis". Journal of Hepatology 36 (3): 321–7. March 2002. doi:10.1016/S0168-8278(01)00288-4. PMID 11867174.
- "Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study". Hepatology 27 (2): 311–6. February 1998. doi:10.1002/hep.510270201. PMID 9462625.
- "Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis". American Journal of Gastroenterology 99 (3): 523–6. March 2004. doi:10.1111/j.1572-0241.2004.04067.x. PMID 15056096.
- ↑ 15.0 15.1 15.2 "Cholangiocarcinoma complicating primary sclerosing cholangitis". Annals of Surgery 213 (1): 21–5. January 1991. doi:10.1097/00000658-199101000-00004. PMID 1845927.
- ↑ Labib, Peter L.; Goodchild, George; Pereira, Stephen P. (2019). "Molecular Pathogenesis of Cholangiocarcinoma". BMC Cancer 19 (1): 185. doi:10.1186/s12885-019-5391-0. PMID 30819129.
- ↑ "Liver fluke-associated cholangiocarcinoma". British Journal of Surgery 89 (8): 962–70. August 2002. doi:10.1046/j.1365-2168.2002.02143.x. PMID 12153620.
- ↑ "Liver fluke induces cholangiocarcinoma". PLOS Medicine 4 (7): e201. July 2007. doi:10.1371/journal.pmed.0040201. PMID 17622191.
- ↑ Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. 72. 2010. 305–50. doi:10.1016/S0065-308X(10)72011-X. ISBN 9780123815132.
- ↑ "Risk factors for gallbladder cancer and cholangiocarcinoma: similarities, differences and updates". Journal of Gastrointestinal Cancer 43 (2): 137–47. June 2012. doi:10.1007/s12029-011-9284-y. PMID 21597894.
- ↑ "Clonorchis sinensis and clonorchiasis, an update". Parasitology International 61 (1): 17–24. March 2012. doi:10.1016/j.parint.2011.06.007. PMID 21741496.
- ↑ "The Lawa model: A sustainable, integrated opisthorchiasis control program using the EcoHealth approach in the Lawa Lake region of Thailand". Parasitology International 66 (4): 346–354. August 2017. doi:10.1016/j.parint.2016.11.013. PMID 27890720.
- ↑ "Incidence of primary cholangiocellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis". Cancer 88 (11): 2471–7. June 2000. doi:10.1002/1097-0142(20000601)88:11<2471::AID-CNCR7>3.0.CO;2-T. PMID 10861422.
- ↑ "Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma". Cancer Science 95 (7): 592–5. July 2004. doi:10.1111/j.1349-7006.2004.tb02492.x. PMID 15245596.
- ↑ "Detection of hepatitis C virus RNA sequences in cholangiocarcinomas in Chinese and American patients". Chinese Medical Journal 113 (12): 1138–41. December 2000. PMID 11776153.
- ↑ 26.0 26.1 26.2 "Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study". Gastroenterology 128 (3): 620–6. March 2005. doi:10.1053/j.gastro.2004.12.048. PMID 15765398.
- ↑ "Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark". Hepatology 28 (4): 921–5. October 1998. doi:10.1002/hep.510280404. PMID 9755226.
- ↑ "Role of bacteria in oncogenesis". Clinical Microbiology Reviews 23 (4): 837–57. October 2010. doi:10.1128/CMR.00012-10. PMID 20930075.
- ↑ "Choledochal cyst disease. A changing pattern of presentation". Annals of Surgery 220 (5): 644–52. November 1994. doi:10.1097/00000658-199411000-00007. PMID 7979612.
- ↑ "Caroli's Disease: a premalignant condition?". American Journal of Surgery 145 (1): 41–8. January 1983. doi:10.1016/0002-9610(83)90164-2. PMID 6295196.
- ↑ "The association between cholangiocarcinoma and hereditary nonpolyposis colorectal carcinoma". Cancer 69 (5): 1112–4. March 1992. doi:10.1002/cncr.2820690508. PMID 1310886.
- ↑ "Clinicopathologic review of 58 patients with biliary papillomatosis". Cancer 100 (4): 783–93. February 2004. doi:10.1002/cncr.20031. PMID 14770435.
- ↑ "What is the impact of coexistence of hepatolithiasis on cholangiocarcinoma?". Journal of Gastroenterology and Hepatology 17 (9): 1015–20. September 2002. doi:10.1046/j.1440-1746.2002.02779.x. PMID 12167124.
- ↑ "Hepatolithiasis associated with cholangiocarcinoma". British Journal of Surgery 84 (7): 969–73. July 1997. doi:10.1002/bjs.1800840717. PMID 9240138.
- ↑ "Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy". Cancer Causes & Control 12 (10): 959–64. December 2001. doi:10.1023/A:1013747228572. PMID 11808716.
- ↑ "Thorotrast-induced cholangiocarcinoma: case report". Abdominal Imaging 28 (1): 72–4. 2003. doi:10.1007/s00261-001-0148-y. PMID 12483389.
- ↑ "Cholangiocarcinoma in association with Thorotrast exposure". Journal of Hepato-Biliary-Pancreatic Surgery 11 (6): 430–3. 2004. doi:10.1007/s00534-004-0924-5. PMID 15619021.
- ↑ "Thorotrast-induced liver neoplasia: a collective review". Journal of the American College of Surgeons 195 (5): 713–8. November 2002. doi:10.1016/S1072-7515(02)01287-5. PMID 12437262.
- ↑ "Adenocarcinoma of the Hepatic Duct at Its Bifurcation Within The Porta Hepatis. An Unusual Tumor With Distinctive Clinical And Pathological Features". American Journal of Medicine 38 (2): 241–56. February 1965. doi:10.1016/0002-9343(65)90178-6. PMID 14256720.
- ↑ "Liver stem cells and their implication in hepatocellular and cholangiocarcinoma". Oncogene 25 (27): 3818–22. June 2006. doi:10.1038/sj.onc.1209558. PMID 16799623.
- ↑ "Possible stem cell origin of human cholangiocarcinoma". World Journal of Gastroenterology 10 (22): 3374–6. November 2004. doi:10.3748/wjg.v10.i22.3374. PMID 15484322.
- ↑ "Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma". American Journal of Pathology 134 (6): 1347–63. June 1989. PMID 2474256.
- ↑ 43.0 43.1 "Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy". Hepatology 41 (1): 5–15. January 2005. doi:10.1002/hep.20537. PMID 15690474.
- ↑ "Mechanisms of biliary carcinogenesis: a pathogenetic multi-stage cascade towards cholangiocarcinoma". Annals of Oncology 10 (Suppl 4): 122–6. 1999. doi:10.1023/A:1008321710719. PMID 10436802.
- ↑ "Cholangiocarcinoma: current concepts and insights". Hepatology 37 (5): 961–9. May 2003. doi:10.1053/jhep.2003.50200. PMID 12717374.
- ↑ "Biliary tract cancers". New England Journal of Medicine 341 (18): 1368–78. October 1999. doi:10.1056/NEJM199910283411807. PMID 10536130.
- ↑ 47.0 47.1 "Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates". Cancer 70 (6): 1498–501. September 1992. doi:10.1002/1097-0142(19920915)70:6<1498::AID-CNCR2820700609>3.0.CO;2-C. PMID 1516001.
- ↑ Studies of the performance of serum markers for cholangiocarcinoma (such as carcinoembryonic antigen and CA19-9) in patients with and without primary sclerosing cholangitis include the following:
- "Serum and bile markers for cholangiocarcinoma". Seminars in Liver Disease 24 (2): 139–54. May 2004. doi:10.1055/s-2004-828891. PMID 15192787.
- "Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis". Gastrointestinal Endoscopy 56 (1): 40–7. July 2002. doi:10.1067/mge.2002.125105. PMID 12085033.
- "The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis". Digestive Diseases and Sciences 50 (9): 1734–40. September 2005. doi:10.1007/s10620-005-2927-8. PMID 16133981.
- "The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis". American Journal of Gastroenterology 95 (1): 204–7. January 2000. doi:10.1111/j.1572-0241.2000.01685.x. PMID 10638584.
- ↑ "Imaging of the hepatobiliary tract". New England Journal of Medicine 336 (26): 1889–94. June 1997. doi:10.1056/NEJM199706263362607. PMID 9197218.
- ↑ "Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective". Tropical Gastroenterology 20 (4): 167–9. 1999. PMID 10769604.
- ↑ "Role of US in the detection, characterization, and staging of cholangiocarcinoma". Radiographics 19 (5): 1199–218. 1999. doi:10.1148/radiographics.19.5.g99se081199. PMID 10489176.
- ↑ "Intrahepatic peripheral cholangiocarcinoma: CT evaluation". Abdominal Imaging 25 (5): 490–6. 2000. doi:10.1007/s002610000079. PMID 10931983.
- ↑ "Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma". AJR. American Journal of Roentgenology 171 (3): 651–8. September 1998. doi:10.2214/ajr.171.3.9725291. PMID 9725291.
- ↑ "Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI". Journal of Computer Assisted Tomography 23 (5): 670–7. 1999. doi:10.1097/00004728-199909000-00004. PMID 10524843.
- ↑ "Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography". Abdominal Imaging 22 (4): 434–8. 1997. doi:10.1007/s002619900227. PMID 9157867.
- ↑ "Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography". AJR. American Journal of Roentgenology 170 (6): 1491–5. June 1998. doi:10.2214/ajr.170.6.9609160. PMID 9609160.
- ↑ "Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures". Gut 46 (1): 103–6. January 2000. doi:10.1136/gut.46.1.103. PMID 10601064.
- ↑ "Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography". World Journal of Surgery 27 (3): 278–83. March 2003. doi:10.1007/s00268-002-6701-1. PMID 12607051.
- ↑ "Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings". American Journal of Gastroenterology 95 (2): 432–40. February 2000. doi:10.1111/j.1572-0241.2000.01763.x. PMID 10685746.
- ↑ "A modern approach to malignant hilar biliary obstruction". Reviews in Gastroenterological Disorders 3 (4): 187–201. 2003. PMID 14668691.
- ↑ "Preoperative imaging of biliary tract cancers". Surgical Oncology Clinics of North America 11 (4): 865–76. October 2002. doi:10.1016/S1055-3207(02)00032-7. PMID 12607576.
- ↑ "Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients". Annals of Surgery 235 (3): 392–9. March 2002. doi:10.1097/00000658-200203000-00011. PMID 11882761.
- ↑ "Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy". Journal of the American College of Surgeons 185 (1): 33–9. July 1997. doi:10.1016/s1072-7515(97)00003-3. PMID 9208958.
- ↑ Image by
Mikael Häggström, MD. Source for caption:
- Nat Pernick, M.D.. "Cytokeratin 19 (CK19, K19)". https://www.pathologyoutlines.com/topic/stainsck19.html. Last author update: 1 October 2013 - ↑ "[The importance of immunohistochemistry for the diagnosis of cholangiocarcinomas]" (in German). Der Pathologe 27 (4): 244–50. July 2006. doi:10.1007/s00292-006-0836-z. PMID 16758167.
- ↑ Darwin PE, Kennedy A. Cholangiocarcinoma at eMedicine
- ↑ "Stage does not predict survival after resection of hilar cholangiocarcinomas promoting an aggressive operative approach". American Journal of Surgery 190 (5): 810–5. November 2005. doi:10.1016/j.amjsurg.2005.07.025. PMID 16226963.
- ↑ "Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience". Annals of Surgery 232 (2): 166–74. August 2000. doi:10.1097/00000658-200008000-00003. PMID 10903592.
- ↑ "Gallbladder and biliary tract carcinoma: A comprehensive update, Part 1". Oncology 18 (7): 889–96. June 2004. PMID 15255172.
- ↑ "Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma". Annals of Surgery 223 (4): 384–94. April 1996. doi:10.1097/00000658-199604000-00007. PMID 8633917.
- ↑ "Surgery for cholangiocarcinoma: the role of liver transplantation". HPB 10 (3): 186–9. 2008. doi:10.1080/13651820801992542. PMID 18773052.
- ↑ "Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma". Transplantation 82 (12): 1703–7. December 2006. doi:10.1097/01.tp.0000253551.43583.d1. PMID 17198263.
- ↑ "Locoregional therapy for cholangiocarcinoma". Current Opinion in Gastroenterology 29 (3): 324–8. May 2013. doi:10.1097/MOG.0b013e32835d9dea. PMID 23337933.
- ↑ "Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma". International Journal of Radiation Oncology, Biology, Physics 46 (3): 581–7. February 2000. doi:10.1016/S0360-3016(99)00472-1. PMID 10701737.
- ↑ "The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer". International Journal of Radiation Oncology, Biology, Physics 28 (4): 945–51. March 1994. doi:10.1016/0360-3016(94)90115-5. PMID 8138448.
- ↑ "Role of radiotherapy, in particular intraluminal brachytherapy, in the treatment of proximal bile duct carcinoma". Annals of Oncology 10 (Suppl 4): 215–20. 1999. doi:10.1023/A:1008339709327. PMID 10436826.
- ↑ "Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival". Annals of Surgery 221 (6): 788–97; discussion 797–8. June 1995. doi:10.1097/00000658-199506000-00017. PMID 7794082.
- ↑ Luvira, V; Satitkarnmanee, E; Pugkhem, A; Kietpeerakool, C; Lumbiganon, P; Pattanittum, P (13 September 2021). "Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma.". The Cochrane Database of Systematic Reviews 2021 (9): CD012814. doi:10.1002/14651858.CD012814.pub2. PMID 34515993.
- ↑ "Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma". Cancer 95 (8): 1685–95. October 2002. doi:10.1002/cncr.10831. PMID 12365016.
- ↑ "National Comprehensive Cancer Network (NCCN) guidelines on evaluation and treatment of hepatobiliary malignancies". https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
- ↑ 81.0 81.1 "NCCN Guidelines for Patients: Gallbladder and Bile Duct Cancers; Hepatobiliary Cancers". National Comprehensive Cancer Network. 2021. https://www.nccn.org/patients/guidelines/content/PDF/gallandbile-hp-patient.pdf.
- ↑ 82.0 82.1 "Recent advances in the management of cholangiocarcinomas". Seminars in Liver Disease 14 (2): 109–14. May 1994. doi:10.1055/s-2007-1007302. PMID 8047893.
- ↑ "Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer". Annals of Oncology 7 (6): 593–600. August 1996. doi:10.1093/oxfordjournals.annonc.a010676. PMID 8879373.
- ↑ "Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas". American Journal of Clinical Oncology 23 (4): 425–8. August 2000. doi:10.1097/00000421-200008000-00023. PMID 10955877.
- ↑ "Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study". Japanese Journal of Clinical Oncology 35 (2): 68–73. February 2005. doi:10.1093/jjco/hyi021. PMID 15709089.
- ↑ "Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM)". Annals of Oncology 17 (Suppl 7): vii73–7. June 2006. doi:10.1093/annonc/mdl956. PMID 16760299.
- ↑ "Gemcitabine and irinotecan in locally advanced or metastatic biliary cancer: preliminary report". Oncology 17 (9 Suppl 8): 23–6. September 2003. PMID 14569844.
- ↑ "Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial". Journal of Clinical Oncology 23 (10): 2332–8. April 2005. doi:10.1200/JCO.2005.51.008. PMID 15800324.
- ↑ "Phase II study of erlotinib in patients with advanced biliary cancer". Journal of Clinical Oncology 24 (19): 3069–74. July 2006. doi:10.1200/JCO.2005.05.3579. PMID 16809731.
- ↑ "Is adjuvant radiotherapy needed after curative resection of extrahepatic biliary tract cancers? A systematic review with a meta-analysis of observational studies". Cancer Treat Rev. 38 (2): 111–119. 2012. doi:10.1016/j.ctrv.2011.05.003. PMID 21652148.
- ↑ 91.0 91.1 "BridgeBio Pharma's Affiliate QED Therapeutics and Partner Helsinn Group Announce FDA Approval of Truseltiq (infigratinib) for Patients with Cholangiocarcinoma" (Press release). BridgeBio Pharma. 28 May 2021. Retrieved 28 May 2021 – via GlobeNewswire.
- ↑ "Pemazyre Prescribing Information". 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213736s000lbl.pdf.
- ↑ Research, Center for Drug Evaluation and (2022-02-01). "FDA approves ivosidenib for advanced or metastatic cholangiocarcinoma" (in en). FDA. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-advanced-or-metastatic-cholangiocarcinoma.
- ↑ "Imfinzi plus chemotherapy reduced risk of death by 20% in 1st-line advanced biliary tract cancer" (in en). https://www.astrazeneca.com/media-centre/press-releases/2022/imfinzi-plus-chemotherapy-reduced-risk-of-death-by-20-in-1st-line-advanced-biliary-tract-cancer.html.
- ↑ "FDA Approves Taiho's Lytgobi (futibatinib) Tablets for Previously Treated, Unresectable, Locally Advanced or Metastatic Intrahepatic Cholangiocarcinoma" (Press release). Taiho Oncology. 30 September 2022. Retrieved 4 October 2022 – via PR Newswire.
- ↑ "Lymph node metastasis in intrahepatic cholangiocarcinoma". Japanese Journal of Clinical Oncology 29 (3): 147–50. March 1999. doi:10.1093/jjco/29.3.147. PMID 10225697.
- ↑ "'Natural history' of unresected cholangiocarcinoma: patient outcome after noncurative intervention". Mayo Clinic Proceedings 70 (5): 425–9. May 1995. doi:10.4065/70.5.425. PMID 7537346.
- ↑ "Role of radiation after operative palliation in cancer of the proximal bile ducts". American Journal of Surgery 161 (4): 454–8. April 1991. doi:10.1016/0002-9610(91)91111-U. PMID 1709795.
- ↑ Studies of surgical outcomes in distal cholangiocarcinoma include:
- "Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors". Annals of Surgery 224 (4): 463–73; discussion 473–5. October 1996. doi:10.1097/00000658-199610000-00005. PMID 8857851.
- "Actual long-term outcome of extrahepatic bile duct cancer after surgical resection". Annals of Surgery 241 (1): 77–84. January 2005. doi:10.1097/01.sla.0000150166.94732.88. PMID 15621994.
- "Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection". Digestive Surgery 17 (1): 36–41. 2000. doi:10.1159/000018798. PMID 10720830.
- "Outcome of treatment for distal bile duct cancer". British Journal of Surgery 83 (12): 1712–5. December 1996. doi:10.1002/bjs.1800831217. PMID 9038548.
- ↑ Studies of outcome in intrahepatic cholangiocarcinoma include:
- "Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors". Annals of Surgery 224 (4): 463–73; discussion 473–5. October 1996. doi:10.1097/00000658-199610000-00005. PMID 8857851.
- "Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience". Journal of Hepato-Biliary-Pancreatic Surgery 5 (1): 41–7. 1998. doi:10.1007/PL00009949. PMID 9683753.
- "Resection of intrahepatic cholangiocarcinoma: a Western experience". Journal of Hepato-Biliary-Pancreatic Surgery 6 (2): 122–7. 1999. doi:10.1007/s005340050094. PMID 10398898.
- "Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma". World Journal of Surgery 27 (3): 289–93. March 2003. doi:10.1007/s00268-002-6696-7. PMID 12607053.
- "Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes". Journal of the American College of Surgeons 193 (4): 384–91. October 2001. doi:10.1016/S1072-7515(01)01016-X. PMID 11584966.
- ↑ Estimates of survival after surgery for perihilar cholangiocarcinoma include:
- "Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system". Annals of Surgery 228 (3): 385–94. September 1998. doi:10.1097/00000658-199809000-00011. PMID 9742921.
- "Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience". Annals of Surgery 232 (2): 166–74. August 2000. doi:10.1097/00000658-200008000-00003. PMID 10903592.
- "Hilar cholangiocarcinoma: a review and commentary". Annals of Surgical Oncology 7 (1): 55–66. 2000. doi:10.1007/s10434-000-0055-4. PMID 10674450.
- "Aggressive surgical resection for cholangiocarcinoma". Archives of Surgery 130 (3): 270–6. March 1995. doi:10.1001/archsurg.1995.01430030040006. PMID 7534059.
- "Segmental liver resections for hilar cholangiocarcinoma". Hepato-Gastroenterology 45 (19): 7–13. 1998. PMID 9496478.
- "Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients". Archives of Surgery 139 (5): 514–23; discussion 523–5. May 2004. doi:10.1001/archsurg.139.5.514. PMID 15136352.
- "Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers". Journal of Hepato-Biliary-Pancreatic Surgery 7 (2): 128–34. 2000. doi:10.1007/s005340050166. PMID 10982604.
- ↑ "Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience". American Journal of Gastroenterology 96 (4): 1164–9. April 2001. doi:10.1111/j.1572-0241.2001.03696.x. PMID 11316165.
- ↑ "Improved survival in resected biliary malignancies". Surgery 132 (4): 555–63; discission 563–4. October 2002. doi:10.1067/msy.2002.127555. PMID 12407338.
- ↑ "Changing international trends in mortality rates for liver, biliary and pancreatic tumours". Journal of Hepatology 37 (6): 806–13. December 2002. doi:10.1016/S0168-8278(02)00297-0. PMID 12445422.
- ↑ "Cancer statistics, 1998". CA: A Cancer Journal for Clinicians 48 (1): 6–29. 1998. doi:10.3322/canjclin.48.1.6. PMID 9449931.
- ↑ Cancer Statistics Home Page — National Cancer Institute
- ↑ Multiple independent studies have documented a steady increase in the worldwide incidence of cholangiocarcinoma. Some relevant journal articles include:
- "Worldwide trends in mortality from biliary tract malignancies". BMC Cancer 2: 10. May 2002. doi:10.1186/1471-2407-2-10. PMID 11991810.
- "Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States". Hepatology 33 (6): 1353–7. June 2001. doi:10.1053/jhep.2001.25087. PMID 11391522.
- "Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?". Journal of Hepatology 40 (3): 472–7. March 2004. doi:10.1016/j.jhep.2003.11.030. PMID 15123362.
- "Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001". British Journal of Cancer 94 (11): 1751–8. June 2006. doi:10.1038/sj.bjc.6603127. PMID 16736026.
- "Changing international trends in mortality rates for liver, biliary and pancreatic tumours". Journal of Hepatology 37 (6): 806–13. December 2002. doi:10.1016/S0168-8278(02)00297-0. PMID 12445422.
- "Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States". Journal of the National Cancer Institute 98 (12): 873–5. June 2006. doi:10.1093/jnci/djj234. PMID 16788161.
- ↑ Table 37.2 in: Sternberg, Stephen (2012). Sternberg's diagnostic surgical pathology. Place of publication not identified: LWW. ISBN 978-1-4511-5289-0. OCLC 953861627.
External links
- American Cancer Society Detailed Guide to Bile Duct Cancer.
- Patient information on extrahepatic bile duct tumors, from the National Cancer Institute.
- Cancer.Net: Bile Duct Cancer
- Cholangiocarcinoma Foundation
- World Cholangiocarcinoma Day
| Classification | |
|---|---|
| External resources |
 |