Biology:Superoxide dismutase mimetics
Superoxide dismutase (SOD) mimetics are synthetic compounds that mimic the native superoxide dismutase enzyme.[1] SOD mimetics effectively convert the superoxide anion (O−2), a reactive oxygen species, into hydrogen peroxide, which is further converted into water by catalase.[2] Reactive oxygen species are natural byproducts of cellular respiration and cause oxidative stress and cell damage, which has been linked to causing cancers, neurodegeneration, age-related declines in health, and inflammatory diseases.[3][4] SOD mimetics are a prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme.[3][5][6]
The chemical structure of SOD mimetics generally consists of manganese, iron, or copper (and zinc) coordination complexes.[1][3][7] Salen-manganese(III) complexes contain aromatic ring structures that increase the lipid solubility and cell permeability of the entire complex.[2] Manganese (II) and iron (III) complexes are commonly used due to their high kinetic and thermodynamic stability, increasing the half-life of the mimetic.[1] However, manganese-based SOD mimetics are found to be more therapeutically effective than their counterparts due to their low toxicity, higher catalytic activity, and increased stability in vivo.[1][3][7]
Mechanism of action
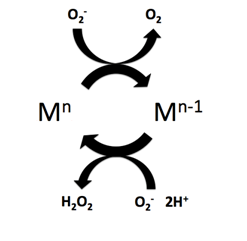
Similar to the native enzyme’s mechanism,[8] the manganese complexes undergo a reversible oxidation/reduction cycle.[2] In the first half reaction manganese covalently coordinates to the superoxide anion on its oxygen binding site,[2] through inner-sphere electron transfer.[3] (Mn) is reduced by superoxide, yielding molecular oxygen and a reduced form of manganese (Mn-1). The metal (Mn-1) is then regenerated to its former oxidation state (Mn) by reducing a second superoxide molecule to hydrogen peroxide.[9]
- 1. Mn + O−2 → Mn-1 + O2
- 2. Mn-1 + O−2 + 2H+ → Mn + H2O2
- Net: Mn + 2O−2 + 2H+ → Mn + O2 + H2O2
The metal complex must be electron deficient in nature, allowing it to accept electrons from the superoxide.[10] This is accomplished by coordinating electron-withdrawing ligands around the metal center.[10] Since the mechanism of SOD mimetics involves a redox cycle, the catalytic activity of the SOD mimetic is partially dependent on the reduction potential of the metal center.[9] Coordinated ligands of SOD mimetics fine-tune the chemical properties of the complex[3] and are designed to match the 300mV reduction potential of the native enzyme.[11]
Manganese-based SODs
The most prominent SOD mimetics are: manganese porphyrin complexes, manganese (II) penta-azamacrocyclic complexes, and manganese (III) salen complexes.[4]
Manganese porphyrin

Porphyrin SOD mimetics consist of manganese (III) centers coordinated by a single porphyrin ring.[10] Although both complexes are effective porphyrin-based superoxide dismutases, MnTBAP [Mn(III)tetrakis (4-benzoic acid) porphyrin] was shown to better protect the cells from oxidative damages compared to ZnTBAP ((Zinc (III) tetrakis (4-benzoic acid)porphyrin chloride)) in vivo.[7] Researchers found MnTBAP reversed obesity[12] and induced faster wound healing in diabetic mice.[13] MnTBAP has the ability to prevent formation of cytotoxic peroxynitrite,[14] a hazardous byproduct of superoxide reacting with nitric oxide, and induces healing process of wounds.[13] MnTMPyP [manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin], another porphyrin molecule, was also found effective in relieving oxidative stress caused by peroxynitrite in intracellular and extracellular conditions.[15] Manganese-porphyrin complexes reduced the damaging effects of radiation treatment in mice.[4]
Manganese (II) penta-azamacrocyclic: M40401/3
M40403 and M40401 are Manganese (II) Penta-Azamacrocyclic complexes with SOD mimetic properties.[16] Mn (II) complexes are found to be more stable in vivo and have high specificity for the superoxide anion, preventing unwanted interactions with biologically important molecules.[1] They are characterized as having a small size, high stability, and higher catalytic efficiency than superoxide dismutase, especially in more acidic environments.[1][16] M40403 was found effective in reducing oxidative tissue damage induced by total body irradiation.[16] M40401 is similar in structure to M40403, but it has two additional methyl groups, causing a one hundredfold increase in catalytic activity in treatment of ischemia-reperfusion injuries.[17] M40401 was also found to protect against hypoxic-ischemic brain injury.[6]
Manganese (III) salen
Mn (III) Salen complexes are found to be more stable than other iron or manganese mimics of superoxide dismutase.[2] In certain synthesized forms, aromatic rings are coordinated with the manganese center, increasing the lipid solubility of the entire complex, allowing it to pass the cellular membrane.[2]
Life-span extension
Treatment of the nematode Caenorhabditis elegans with superoxide dismutase/catalase (SOD/catalase) mimetics has been reported to extend life-span.[18][19] Mice with deficient SOD2 die prematurely, exhibiting severe metabolic and mitochondrial defects. Treatment of such mice with SOD/catalase mimetics extended their life-span by as much as three-fold.[20] Treatment of wild-type mice with a carboxyfullerene SOD mimetic not only reduced age-associated oxidative stress and mitochondrial radical production, but significantly extended life-span.[5] This treatment also rescued age-related cognitive impairment. These findings suggest that oxidative stress is an important determinant of life-span.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Superoxide Dismutase Mimetics". Pulmonary Pharmacology & Therapeutics 15 (5): 439–447. 2002. doi:10.1006/pupt.2002.0374. PMID 12406666.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Baudry, M; Etienne, S; Bruce, A; Palucki, M; Jacobsen, E; Malfroy, B (30 April 1993). "Salen-Manganese Complexes Are Superoxide Dismutase-Mimics". Biochemical and Biophysical Research Communications 192 (2): 964–68. Retrieved 10 January 2015.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Comparative studies on manganese-based SOD mimetics, including the phosphate effect, by using global spectral analysis". Journal of Inorganic Biochemistry 109: 26–32. 2012. doi:10.1016/j.jinorgbio.2011.12.008. PMID 22366231.
- ↑ 4.0 4.1 4.2 Vujaskovic, Zeljko; Batinic-Haberle, Ines; Rabbani, Zahid N; Feng, Qin-fu; Kang, Song K; Spasojevic, Ivan; Samulski, Thaddeus V; Fridovich, Irwin et al. (2002). "A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury". Free Radical Biology and Medicine (Elsevier BV) 33 (6): 857–863. doi:10.1016/s0891-5849(02)00980-2. ISSN 0891-5849. PMID 12208373.
- ↑ 5.0 5.1 "A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice". Neurobiol. Aging 29 (1): 117–28. January 2008. doi:10.1016/j.neurobiolaging.2006.09.014. PMID 17079053.
- ↑ 6.0 6.1 "Neuroprotection against hypoxia-ischemia in neonatal rat brain by novel superoxide dismutase mimetics". Neuroscience Letters 346 (1–2): 41–4. 2003. doi:10.1016/S0304-3940(03)00558-5. PMID 12850543.
- ↑ 7.0 7.1 7.2 "A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro.". J Pharmacol Exp Ther 275 (3): 1227–32. 1995. PMID 8531085.
- ↑ "Superoxide dismutases: active sites that save, but a protein that kills". Current Opinion in Chemical Biology 8 (2): 162–68. 2004. doi:10.1016/j.cbpa.2004.02.011. PMID 15062777.
- ↑ 9.0 9.1 "Impact of electrostatics in redox modulation of oxidative stress by Mn porphyrins: Protection of SOD-deficient Escherichia coli via alternative mechanism where Mn porphyrin acts as a Mn carrier". Free Radical Biology and Medicine 45 (2): 201–10. 2008. doi:10.1016/j.freeradbiomed.2008.04.009. PMID 18457677.
- ↑ 10.0 10.1 10.2 "Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics". Free Radical Biology and Medicine 51 (5): 1035–53. 2011. doi:10.1016/j.freeradbiomed.2011.04.046. PMID 21616142.
- ↑ Crapo, James; Day, Brian; Fridovich, Irwin. "Development of Manganic Porphyrin Mimetics of Superoxide Dismutase Activity". Madame Curie Bioscience Database [Internet]. Landes Bioscience. Retrieved 31 January 2015.
- ↑ "A new class of anti-obesity compounds with potential anti-diabetic properties". 17 April 2012. https://www.kurzweilai.net/a-new-class-of-anti-obesity-compounds-with-potential-anti-diabetic-properties.
- ↑ 13.0 13.1 "Therapeutic administration of superoxide dismutase (SOD) mimetics normalizes wound healing in diabetic mice". Journal of the American College of Surgeons 201 (3): S57. 2005. doi:10.1016/j.jamcollsurg.2005.06.124.
- ↑ "Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-induced pleurisy". Free Radical Biology and Medicine 26 (1–2): 25–33. 1999. doi:10.1016/s0891-5849(98)00142-7. PMID 9890637.
- ↑ "Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics". British Journal of Pharmacology 124 (4): 719–28. 1998. doi:10.1038/sj.bjp.0701899. PMID 9690864.
- ↑ 16.0 16.1 16.2 "The manganese superoxide dismutase mimetic, M40403, protects adult mice from lethal total body irradiation". Free Radical Research 44 (5): 529–40. 2010. doi:10.3109/10715761003649578. PMID 20298121.
- ↑ "Protective effects of a new stable, highly active SOD mimetic, M40401in splanchnic artery occlusion and reperfusion". British Journal of Pharmacology 132 (1): 19–29. 2001. doi:10.1038/sj.bjp.0703775. PMID 11156557.
- ↑ "Extension of life-span with superoxide dismutase/catalase mimetics". Science 289 (5484): 1567–9. September 2000. doi:10.1126/science.289.5484.1567. PMID 10968795. Bibcode: 2000Sci...289.1567M.
- ↑ "Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans". Mech. Ageing Dev. 129 (6): 322–31. June 2008. doi:10.1016/j.mad.2008.02.011. PMID 18400258.
- ↑ "Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics". J. Neurosci. 21 (21): 8348–53. November 2001. doi:10.1523/jneurosci.21-21-08348.2001. PMID 11606622.
 |