Biology:Catalase
| Catalase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Catalase | ||||||||
| Pfam | PF00199 | ||||||||
| InterPro | IPR011614 | ||||||||
| PROSITE | PDOC00395 | ||||||||
| SCOP2 | 7cat / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 370 | ||||||||
| OPM protein | 3e4w | ||||||||
| CDD | cd00328 | ||||||||
| |||||||||
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen.[1] It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.[2]
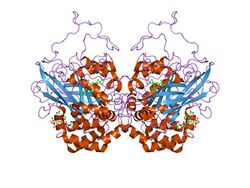
Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long.[3] It contains four iron-containing heme groups that allow the enzyme to react with hydrogen peroxide. The optimum pH for human catalase is approximately 7,[4] and has a fairly broad maximum: the rate of reaction does not change appreciably between pH 6.8 and 7.5.[5] The pH optimum for other catalases varies between 4 and 11 depending on the species.[6] The optimum temperature also varies by species.[7]
Structure
Human catalase forms a tetramer composed of four subunits, each of which can be conceptually divided into four domains.[8] The extensive core of each subunit is generated by an eight-stranded antiparallel β-barrel (β1-8), with nearest neighbor connectivity capped by β-barrel loops on one side and α9 loops on the other.[8] A helical domain at one face of the β-barrel is composed of four C-terminal helices (α16, α17, α18, and α19) and four helices derived from residues between β4 and β5 (α4, α5, α6, and α7).[8] Alternative splicing may result in different protein variants.
History
Catalase was first noticed in 1818 by Louis Jacques Thénard, who discovered hydrogen peroxide (H2O2). Thénard suggested its breakdown was caused by an unknown substance. In 1900, Oscar Loew was the first to give it the name catalase, and found it in many plants and animals.[9] In 1937 catalase from beef liver was crystallized by James B. Sumner and Alexander Dounce[10] and the molecular weight was measured in 1938.[11]
The amino acid sequence of bovine catalase was determined in 1969,[12] and the three-dimensional structure in 1981.[13]
Function
Molecular mechanism
While the complete mechanism of catalase is not currently known,[14] the reaction is believed to occur in two stages:
- H2O2 + Fe(III)-E → H2O + O=Fe(IV)-E(.+)
- H2O2 + O=Fe(IV)-E(.+) → H2O + Fe(III)-E + O2[14]
Here Fe()-E represents the iron center of the heme group attached to the enzyme. Fe(IV)-E(.+) is a mesomeric form of Fe(V)-E, meaning the iron is not completely oxidized to +V, but receives some stabilising electron density from the heme ligand, which is then shown as a radical cation (.+).
As hydrogen peroxide enters the active site, it does interact with the amino acids Asn148 (asparagine at position 148) and His75, causing a proton (hydrogen ion) to transfer between the oxygen atoms. The free oxygen atom coordinates, freeing the newly formed water molecule and Fe(IV)=O. Fe(IV)=O reacts with a second hydrogen peroxide molecule to reform Fe(III)-E and produce water and oxygen.[14] The reactivity of the iron center may be improved by the presence of the phenolate ligand of Tyr358 in the fifth coordination position, which can assist in the oxidation of the Fe(III) to Fe(IV). The efficiency of the reaction may also be improved by the interactions of His75 and Asn148 with reaction intermediates.[14] The decomposition of hydrogen peroxide by catalase proceeds according to first-order kinetics, the rate being proportional to the hydrogen peroxide concentration.[15]
Catalase can also catalyze the oxidation, by hydrogen peroxide, of various metabolites and toxins, including formaldehyde, formic acid, phenols, acetaldehyde and alcohols. It does so according to the following reaction:
- H2O2 + H2R → 2H2O + R
The exact mechanism of this reaction is not known.
Any heavy metal ion (such as copper cations in copper(II) sulfate) can act as a noncompetitive inhibitor of catalase. However, "Copper deficiency can lead to a reduction in catalase activity in tissues, such as heart and liver."[16] Furthermore, the poison cyanide is a noncompetitive inhibitor[17] of catalase at high concentrations of hydrogen peroxide.[18] Arsenate acts as an activator.[19] Three-dimensional protein structures of the peroxidated catalase intermediates are available at the Protein Data Bank.
Cellular role
Hydrogen peroxide is a harmful byproduct of many normal metabolic processes; to prevent damage to cells and tissues, it must be quickly converted into other, less dangerous substances. To this end, catalase is frequently used by cells to rapidly catalyze the decomposition of hydrogen peroxide into less-reactive gaseous oxygen and water molecules.[20]
Mice genetically engineered to lack catalase are initially phenotypically normal.[21] However, catalase deficiency in mice may increase the likelihood of developing obesity, fatty liver,[22] and type 2 diabetes.[23] Some humans have very low levels of catalase (acatalasia), yet show few ill effects.
The increased oxidative stress that occurs with aging in mice is alleviated by over-expression of catalase.[24] Over-expressing mice do not exhibit the age-associated loss of spermatozoa, testicular germ and Sertoli cells seen in wild-type mice. Oxidative stress in wild-type mice ordinarily induces oxidative DNA damage (measured as 8-oxodG) in sperm with aging, but these damages are significantly reduced in aged catalase over-expressing mice.[24] Furthermore, these over-expressing mice show no decrease in age-dependent number of pups per litter. Overexpression of catalase targeted to mitochondria extends the lifespan of mice.[25]
In eukaryotes, catalase is usually located in a cellular organelle called the peroxisome.[26] Peroxisomes in plant cells are involved in photorespiration (the use of oxygen and production of carbon dioxide) and symbiotic nitrogen fixation (the breaking apart of diatomic nitrogen (N2) to reactive nitrogen atoms). Hydrogen peroxide is used as a potent antimicrobial agent when cells are infected with a pathogen. Catalase-positive pathogens, such as Mycobacterium tuberculosis, Legionella pneumophila, and Campylobacter jejuni, make catalase to deactivate the peroxide radicals, thus allowing them to survive unharmed within the host.[27]
Like alcohol dehydrogenase, catalase converts ethanol to acetaldehyde, but it is unlikely that this reaction is physiologically significant.[28]
Distribution among organisms
The large majority of known organisms use catalase in every organ, with particularly high concentrations occurring in the liver in mammals.[29] Catalase is found primarily in peroxisomes and the cytosol of erythrocytes (and sometimes in mitochondria[30])
Almost all aerobic microorganisms use catalase. It is also present in some anaerobic microorganisms, such as Methanosarcina barkeri.[31] Catalase is also universal among plants and occurs in most fungi.[32]
One unique use of catalase occurs in the bombardier beetle. This beetle has two sets of liquids that are stored separately in two paired glands. The larger of the pair, the storage chamber or reservoir, contains hydroquinones and hydrogen peroxide, while the smaller, the reaction chamber, contains catalases and peroxidases. To activate the noxious spray, the beetle mixes the contents of the two compartments, causing oxygen to be liberated from hydrogen peroxide. The oxygen oxidizes the hydroquinones and also acts as the propellant.[33] The oxidation reaction is very exothermic (ΔH = −202.8 kJ/mol) and rapidly heats the mixture to the boiling point.[34]
Long-lived queens of the termite Reticulitermes speratus have significantly lower oxidative damage to their DNA than non-reproductive individuals (workers and soldiers).[35] Queens have more than two times higher catalase activity and seven times higher expression levels of the catalase gene RsCAT1 than workers.[35] It appears that the efficient antioxidant capability of termite queens can partly explain how they attain longer life.
Catalase enzymes from various species have vastly differing optimum temperatures. Poikilothermic animals typically have catalases with optimum temperatures in the range of 15-25 °C, while mammalian or avian catalases might have optimum temperatures above 35 °C,[36][37] and catalases from plants vary depending on their growth habit.[36] In contrast, catalase isolated from the hyperthermophile archaeon Pyrobaculum calidifontis has a temperature optimum of 90 °C.[38]
Clinical significance and application
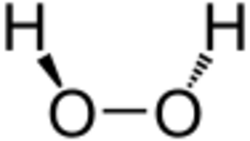
Catalase is used in the food industry for removing hydrogen peroxide from milk prior to cheese production.[39] Another use is in food wrappers, where it prevents food from oxidizing.[40] Catalase is also used in the textile industry, removing hydrogen peroxide from fabrics to make sure the material is peroxide-free.[41]
A minor use is in contact lens hygiene – a few lens-cleaning products disinfect the lens using a hydrogen peroxide solution; a solution containing catalase is then used to decompose the hydrogen peroxide before the lens is used again.[42]
Bacterial identification (catalase test)

The catalase test is one of the three main tests used by microbiologists to identify species of bacteria. If the bacteria possess catalase (i.e., are catalase-positive), bubbles of oxygen are observed when a small amount of bacterial isolate is added to hydrogen peroxide. The catalase test is done by placing a drop of hydrogen peroxide on a microscope slide. An applicator stick is touched to the colony, and the tip is then smeared onto the hydrogen peroxide drop.
- If the mixture produces bubbles or froth, the organism is said to be 'catalase-positive'. Staphylococci[43] and Micrococci[44] are catalase-positive. Other catalase-positive organisms include Listeria, Corynebacterium diphtheriae, Burkholderia cepacia, Nocardia, the family Enterobacteriaceae (Citrobacter, E. coli, Enterobacter, Klebsiella, Shigella, Yersinia, Proteus, Salmonella, Serratia), Pseudomonas, Mycobacterium tuberculosis, Aspergillus, Cryptococcus, and Rhodococcus equi.
- If not, the organism is 'catalase-negative'. Streptococcus[45] and Enterococcus spp. are catalase-negative.
While the catalase test alone cannot identify a particular organism, it can aid identification when combined with other tests such as antibiotic resistance. The presence of catalase in bacterial cells depends on both the growth condition and the medium used to grow the cells.
Capillary tubes may also be used. A small sample of bacteria is collected on the end of the capillary tube, without blocking the tube, to avoid false negative results. The opposite end is then dipped into hydrogen peroxide, which is drawn into the tube through capillary action, and turned upside down, so that the bacterial sample points downwards. The hand holding the tube is then tapped on the bench, moving the hydrogen peroxide down until it touches the bacteria. If bubbles form on contact, this indicates a positive catalase result. This test can detect catalase-positive bacteria at concentrations above about 105 cells/mL,[46] and is simple to use.
Bacterial virulence
Neutrophils and other phagocytes use peroxide to kill bacteria. The enzyme NADPH oxidase generates superoxide within the phagosome, which is converted via hydrogen peroxide to other oxidising substances like hypochlorous acid which kill phagocytosed pathogens.[47] In individuals with chronic granulomatous disease (CGD), phagocytic peroxide production is impaired due to a defective NADPH oxidase system. Normal cellular metabolism will still produce a small amount of peroxide and this peroxide can be used to produce hypochlorous acid to eradicate the bacterial infection. However, if individuals with CGD are infected with catalase-positive bacteria, the bacterial catalase can destroy the excess peroxide before it can be used to produce other oxidising substances. In these individuals the pathogen survives and becomes a chronic infection. This chronic infection is typically surrounded by macrophages in an attempt to isolate the infection. This wall of macrophages surrounding a pathogen is called a granuloma. Many bacteria are catalase positive, but some are better catalase-producers than others. Some catalase-positive bacteria and fungi include: Nocardia, Pseudomonas, Listeria, Aspergillus, Candida, E. coli, Staphylococcus, Serratia, B. cepacia and H. pylori.[48]
Acatalasia
Acatalasia is a condition caused by homozygous mutations in CAT, resulting in a lack of catalase. Symptoms are mild and include oral ulcers. A heterozygous CAT mutation results in lower, but still present catalase.[49]
Gray hair
Low levels of catalase may play a role in the graying process of human hair. Hydrogen peroxide is naturally produced by the body and broken down by catalase. Hydrogen peroxide can accumulate in hair follicles and if catalase levels decline, this buildup can cause oxidative stress and graying.[50] These low levels of catalase are associated with old age. Hydrogen peroxide interferes with the production of melanin, the pigment that gives hair its color.[51][52]
Interactions
Catalase has been shown to interact with the ABL2[53] and Abl genes.[53] Infection with the murine leukemia virus causes catalase activity to decline in the lungs, heart and kidneys of mice. Conversely, dietary fish oil increased catalase activity in the heart, and kidneys of mice.[54]
Methods for determining catalase activity
In 1870, Schoenn discovered a formation of yellow color from the interaction of hydrogen peroxide with molybdate;[55] then, from the middle of the 20th century, this reaction began to be used for colorimetric determination of unreacted hydrogen peroxide in the catalase activity assay.[56] The reaction became widely used after publications by Korolyuk et al. (1988)[57] and Goth (1991).[58] The first paper describes serum catalase assay with no buffer in the reaction medium; the latter describes the procedure based on phosphate buffer as a reaction medium. Since phosphate ion reacts with ammonium molybdate,[58] the use of MOPS buffer as a reaction medium is more appropriate.[59]
Direct UV measurement of the decrease in the concentration of hydrogen peroxide is also widely used after the publications by Beers & Sizer[60] and Aebi.[61]
See also
References
- ↑ "Diversity of structures and properties among catalases". Cellular and Molecular Life Sciences 61 (2): 192–208. January 2004. doi:10.1007/s00018-003-3206-5. PMID 14745498.
- ↑ Goodsell DS (2004-09-01). "Catalase". Molecule of the Month. RCSB Protein Data Bank. http://pdb101.rcsb.org/motm/57.
- ↑ "Catalase: H2O2: H2O2 Oxidoreductase". Catalase Structural Tutorial Text. http://biology.kenyon.edu/BMB/Chime/catalase/frames/cattx.htm.
- ↑ "The assay of catalases and peroxidases". Methods of Biochemical Analysis. 1. 1954. pp. 357–424. doi:10.1002/9780470110171.ch14. ISBN 978-0-470-11017-1.
- ↑ "Catalase in vitro". Oxygen Radicals in Biological Systems. Methods in Enzymology. 105. 1984. pp. 121–6. doi:10.1016/S0076-6879(84)05016-3. ISBN 978-0-12-182005-3. https://archive.org/details/oxygenradicalsin0000unse/page/121.
- ↑ "EC 1.11.1.6 - catalase". BRENDA: The Comprehensive Enzyme Information System. Department of Bioinformatics and Biochemistry, Technische Universität Braunschweig. http://www.brenda-enzymes.org/enzyme.php?ecno=1.11.1.6.
- ↑ "A Quantitative Enzyme Study; CATALASE". bucknell.edu. http://www.facstaff.bucknell.edu/toner/gb/lab121/labs34.html.
- ↑ 8.0 8.1 8.2 "Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism". Journal of Molecular Biology 296 (1): 295–309. February 2000. doi:10.1006/jmbi.1999.3458. PMID 10656833.
- ↑ "A New Enzyme of General Occurrence in Organisms". Science 11 (279): 701–702. May 1900. doi:10.1126/science.11.279.701. PMID 17751716. Bibcode: 1900Sci....11..701L. https://zenodo.org/record/1447826.
- ↑ "Crystalline Catalase". Science 85 (2206): 366–367. April 1937. doi:10.1126/science.85.2206.366. PMID 17776781. Bibcode: 1937Sci....85..366S.
- ↑ "The Molecular Weight of Crystalline Catalase". Science 87 (2256): 284. March 1938. doi:10.1126/science.87.2256.284. PMID 17831682. Bibcode: 1938Sci....87..284S.
- ↑ "The amino acid sequence of bovine liver catalase: a preliminary report". Archives of Biochemistry and Biophysics 131 (2): 653–655. May 1969. doi:10.1016/0003-9861(69)90441-X. PMID 4892021.
- ↑ "Structure of beef liver catalase". Journal of Molecular Biology 152 (2): 465–499. October 1981. doi:10.1016/0022-2836(81)90254-0. PMID 7328661.
- ↑ 14.0 14.1 14.2 14.3 "Proposed Mechanism of Catalase". Catalase: H2O2: H2O2 Oxidoreductase: Catalase Structural Tutorial. http://biology.kenyon.edu/BMB/Chime/catalase/frames/cattx.htm#Proposed%20Mechanism%20of%20Catalase.
- ↑ "Catalase in vitro". Oxygen Radicals in Biological Systems. Methods in Enzymology. 105. 1984. pp. 121–126. doi:10.1016/S0076-6879(84)05016-3. ISBN 978-0-12-182005-3.
- ↑ "The many "faces" of copper in medicine and treatment". Biometals 27 (4): 611–621. August 2014. doi:10.1007/s10534-014-9736-5. PMID 24748564.
- ↑ "Nonstationary inhibition of enzyme action. The cyanide inhibition of catalase.". The Journal of Physical Chemistry 85 (7): 835–839. April 1981. doi:10.1021/j150607a021.
- ↑ "Steady-state kinetics of the catalase reaction in the presence of cyanide". Journal of Biochemistry 94 (2): 403–408. August 1983. doi:10.1093/oxfordjournals.jbchem.a134369. PMID 6630165.
- ↑ "Characterization of glutathione reductase and catalase in the fronds of two Pteris ferns upon arsenic exposure". Plant Physiology and Biochemistry 47 (10): 960–965. October 2009. doi:10.1016/j.plaphy.2009.05.009. PMID 19574057. Bibcode: 2009PlPB...47..960K.
- ↑ "Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes". Blood 87 (4): 1595–1599. February 1996. doi:10.1182/blood.V87.4.1595.bloodjournal8741595. PMID 8608252.
- ↑ "Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury". The Journal of Biological Chemistry 279 (31): 32804–32812. July 2004. doi:10.1074/jbc.M404800200. PMID 15178682.
- ↑ "Catalase deletion promotes prediabetic phenotype in mice". Free Radical Biology & Medicine 103: 48–56. February 2017. doi:10.1016/j.freeradbiomed.2016.12.011. PMID 27939935.
- ↑ "Acatalasemia and diabetes mellitus". Archives of Biochemistry and Biophysics 525 (2): 195–200. September 2012. doi:10.1016/j.abb.2012.02.005. PMID 22365890.
- ↑ 24.0 24.1 "Overexpression of catalase in mice reduces age-related oxidative stress and maintains sperm production". Experimental Gerontology 84: 12–20. November 2016. doi:10.1016/j.exger.2016.08.012. PMID 27575890.
- ↑ "Extension of murine life span by overexpression of catalase targeted to mitochondria". Science 308 (5730): 1909–1911. June 2005. doi:10.1126/science.1106653. PMID 15879174. Bibcode: 2005Sci...308.1909S.
- ↑ "Peroxisomes". Molecular Biology of the Cell (4th ed.). New York: Garland Science. 2002. ISBN 978-0-8153-3218-3. https://www.ncbi.nlm.nih.gov/books/NBK26858/.
- ↑ "A major catalase (KatB) that is required for resistance to H2O2 and phagocyte-mediated killing in Edwardsiella tarda". Microbiology 149 (Pt 9): 2635–2644. September 2003. doi:10.1099/mic.0.26478-0. PMID 12949187.
- ↑ "Ethanol metabolism, cirrhosis and alcoholism". Clinica Chimica Acta; International Journal of Clinical Chemistry 257 (1): 59–84. January 1997. doi:10.1016/S0009-8981(96)06434-0. PMID 9028626.
- ↑ "Superoxide Dismutase and Catalase in the Organs of Mammals of Different Ecogenesis". Journal of Evolutionary Biochemistry and Physiology 37 (3): 241–245. 2001. doi:10.1023/A:1012663105999.
- ↑ "Mitochondrial catalase and oxidative injury". Biological Signals and Receptors 10 (3–4): 189–199. 2001. doi:10.1159/000046887. PMID 11351128.
- ↑ "The catalase and superoxide dismutase genes are transcriptionally up-regulated upon oxidative stress in the strictly anaerobic archaeon Methanosarcina barkeri". Microbiology 152 (Pt 6): 1671–1677. June 2006. doi:10.1099/mic.0.28542-0. PMID 16735730.
- ↑ "Fungal catalases: function, phylogenetic origin and structure". Archives of Biochemistry and Biophysics 525 (2): 170–180. September 2012. doi:10.1016/j.abb.2012.05.014. PMID 22698962.
- ↑ "Spray aiming in the bombardier beetle: photographic evidence". Proceedings of the National Academy of Sciences of the United States of America 96 (17): 9705–9709. August 1999. doi:10.1073/pnas.96.17.9705. PMID 10449758. Bibcode: 1999PNAS...96.9705E.
- ↑ "A biomimetic study of the explosive discharge of the bombardier beetle". International Journal of Design & Nature 1 (1): 1–9. 2006. http://www.heveliusforum.org/Artykuly/Biomimetics.pdf.
- ↑ 35.0 35.1 "An Efficient Antioxidant System in a Long-Lived Termite Queen". PLOS ONE 12 (1). 2017. doi:10.1371/journal.pone.0167412. PMID 28076409. Bibcode: 2017PLoSO..1267412T.
- ↑ 36.0 36.1 "Studies on Catalase". Bulletin of the Institute for Chemical Research, Kyoto University 34 (4): 165–192. 1956-07-31. https://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/75561/1/chd034_4_165.pdf. Retrieved 27 September 2017.
- ↑ "Immobilization of catalase into chemically crosslinked chitosan beads". Enzyme and Microbial Technology 32 (7): 889–894. June 2003. doi:10.1016/S0141-0229(03)00065-6.
- ↑ "Unique presence of a manganese catalase in a hyperthermophilic archaeon, Pyrobaculum calidifontis VA1". Journal of Bacteriology 184 (12): 3305–3312. June 2002. doi:10.1128/JB.184.12.3305-3312.2002. PMID 12029047.
- ↑ "Catalase". Worthington Enzyme Manual. Worthington Biochemical Corporation. http://www.worthington-biochem.com/CTL/default.html.
- ↑ Hengge A (1999-03-16). "Re: how is catalase used in industry?". General Biology. MadSci Network. http://madsci.org/posts/archives/mar99/921636249.Gb.r.html.
- ↑ "textile industry". Case study 228. International Cleaner Production Information Clearinghouse. http://www.p2pays.org/ref/11/10842.htm.
- ↑ US patent 5521091, Cook JN, Worsley JL, "Compositions and method for destroying hydrogen peroxide on contact lens", issued 1996-05-28
- ↑ Rollins DM (2000-08-01). "Bacterial Pathogen List". BSCI 424 Pathogenic Microbiology. University of Maryland. http://www.life.umd.edu/classroom/bsci424/pathogendescriptions/PathogenList.htm.
- ↑ Johnson M. "Catalase Production". Biochemical Tests. Mesa Community College. http://www.mc.maricopa.edu/~johnson/labtools/Dbiochem/cat.html.
- ↑ Fox A. "Streptococcus pneumoniae and Staphylococci". University of South Carolina. http://pathmicro.med.sc.edu/fox/strep-staph.htm.
- ↑ (in en) Fisheries Processing: Biotechnological applications. Springer Science & Business Media. 2012-12-06. ISBN 978-1-4615-5303-8. https://books.google.com/books?id=_orkBwAAQBAJ&pg=PA35.
- ↑ "Reactive Oxygen Species and Neutrophil Function". Annual Review of Biochemistry 85 (1): 765–792. June 2016. doi:10.1146/annurev-biochem-060815-014442. PMID 27050287.
- ↑ First aid for the USMLE step 1 2017: a student-to-student guide (27th ed.). New York: McGraw-Hill Education. 2017-01-06. ISBN 978-1-259-83762-3. OCLC 986222844.
- ↑ "OMIM Entry - # 614097 - ACATALASEMIA" (in en-us). http://www.omim.org/entry/614097.
- ↑ "Gray hair cure? Scientists find root cause of discoloration" (in en). 6 May 2013. http://www.nbcnews.com/healthmain/gray-hair-cure-scientists-find-root-cause-discoloration-6C9802771.
- ↑ "Why Hair Turns Gray Is No Longer A Gray Area: Our Hair Bleaches Itself As We Grow Older". Science News. ScienceDaily. 2009-02-24. https://www.sciencedaily.com/releases/2009/02/090223131123.htm.
- ↑ "Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair". FASEB Journal 23 (7): 2065–2075. July 2009. doi:10.1096/fj.08-125435. PMID 19237503.
- ↑ 53.0 53.1 "Catalase activity is regulated by c-Abl and Arg in the oxidative stress response". The Journal of Biological Chemistry 278 (32): 29667–29675. August 2003. doi:10.1074/jbc.M301292200. PMID 12777400.
- ↑ "Effects of dietary fish oil on tissue glutathione and antioxidant defense enzymes in mice with murine aids". Nutrition Research 20 (9): 1287–99. 2000. doi:10.1016/S0271-5317(00)00214-1.
- ↑ "A colorimetric determination of hydrogen peroxide". Journal of the American Chemical Society 44 (8): 1662–1663. 1922. doi:10.1021/ja01429a006. Bibcode: 1922JAChS..44.1662I. https://pubs.acs.org/doi/pdf/10.1021/ja01429a006.
- ↑ "A colorimetric test for measuring catalase activity of cultures of M. tuberculosis". American Review of Tuberculosis 71 (2): 305–313. 1955. doi:10.1164/artpd.1955.71.2.305. PMID 14350192. https://www.atsjournals.org/doi/abs/10.1164/artpd.1955.71.2.305.
- ↑ "[A method of determining catalase activity]". Laboratornoe Delo (1): 16–19. 1988. PMID 2451064.
- ↑ 58.0 58.1 "A simple method for determination of serum catalase activity and revision of reference range". Clinica Chimica Acta; International Journal of Clinical Chemistry 196 (2–3): 143–151. February 1991. doi:10.1016/0009-8981(91)90067-m. PMID 2029780.
- ↑ "Catalase enzymatic activity in adult mosquitoes (Diptera: Culicidae): taxonomic distribution of the continuous trait suggests its relevance for phylogeny research". Zootaxa 5339 (2): 159–176. 2023. doi:10.11646/zootaxa.5339.2.3. PMID 38221060. https://www.mapress.com/zt/article/view/zootaxa.5339.2.3.
- ↑ "A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase". The Journal of Biological Chemistry 195 (1): 133–140. March 1952. doi:10.1016/S0021-9258(19)50881-X. PMID 14938361.
- ↑ "Catalase in vitro". Oxygen Radicals in Biological Systems. Methods in Enzymology. 105. Academic Press. January 1984. pp. 121–126. doi:10.1016/s0076-6879(84)05016-3. ISBN 978-0-12-182005-3.
External links
- "GenAge entry for CAT (Homo sapiens)". Human Ageing Genomic Resources. http://genomics.senescence.info/genes/entry.php?hugo=CAT.
- "Catalase". MadSci FAQ. madsci.org. http://madsci.org/FAQs/catalase.html.
- "Catalase and oxidase test video". Regnvm Prokaryotae. http://www.tgw1916.net/video_pages/catalase.html.
- "EC 1.11.1.6 - catalase". Brenda: The Comprehensive Enzyme Information System. http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.11.1.6.
- "PeroxiBase - The peroxidase database". Swiss Institute of Bioinformatics. http://peroxibase.isb-sib.ch/.
- "Catalase Procedure". MicrobeID.com. http://microbeid.com/Methods/catalase.html.
- "Catalase Molecule of the Month". Protein Data Bank. http://www.rcsb.org/pdb/101/motm.do?momID=57.html.
- Overview of all the structural information available in the PDB for UniProt: P04040 (Catalase) at the PDBe-KB.
 |
