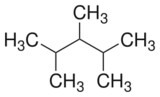
Chemistry:2,3,4-Trimethylpentane
From HandWiki
Revision as of 19:00, 14 November 2021 by imported>BotanyGa (change)
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,3,4-Trimethylpentane[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 1696869 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | 2,3,4-trimethylpentane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3295 |
| |
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.232 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Odourless |
| Density | 719 mg mL−1 |
| Melting point | −109.7 to −109.0 °C; −165.5 to −164.1 °F; 163.4 to 164.2 K |
| Boiling point | 113.4 to 114.0 °C; 236.0 to 237.1 °F; 386.5 to 387.1 K |
| Vapor pressure | 6.7549 kPa (at 37.7 °C) |
Henry's law
constant (kH) |
5.6 nmol Pa−1 kg−1 |
Refractive index (nD)
|
1.404 |
| Thermochemistry | |
Heat capacity (C)
|
247.32 J K−1 mol−1 |
Std molar
entropy (S |
329.32 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−256.9–−253.5 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−5.4671–−5.4639 MJ mol−1 |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | DANGER |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+310, P331 | |
| Flash point | 4 °C (39 °F; 277 K) |
| Explosive limits | 1–?% |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2,3,4-Trimethylpentane is a branched alkane. It is one of the isomers of octane.
References
- ↑ "2,3,4-trimethylpentane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=11269&loc=ec_rcs. Retrieved 12 March 2012.
External links
- 2,3,4-Trimethylpentane at environmentalchemistry.com
 |

