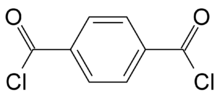
Chemistry:Terephthaloyl chloride
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzene-1,4-dicarbonyl dichloride[1] | |
| Other names
Terephthaloyl dichloride[1]
1,4-Benzenedicarbonyl chloride Benzene-1,4-dicarbonyl chloride Terephthalic acid dichloride Terephthaloyl dichloride p-Phthalyl chloride TCL | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H4Cl2O2 | |
| Molar mass | 203.02 g/mol |
| Appearance | white solid |
| Density | 1.34 g/cm3 |
| Melting point | 81.5 to 83 °C (178.7 to 181.4 °F; 354.6 to 356.1 K) |
| Boiling point | 265 °C (509 °F; 538 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
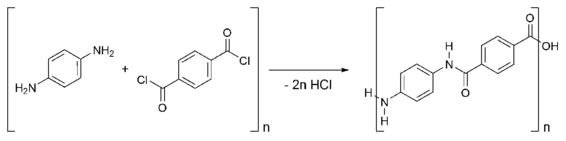
Terephthaloyl chloride (TCL, 1,4-benzenedicarbonyl chloride) is the acyl chloride of terephthalic acid. It is a white solid. It is one of two precursors used to make Kevlar, the other being p-phenylenediamine. TCL is used as a key component in performance polymers and aramid fibers, where it imparts flame resistance, chemical resistance, temperature stability, light weight, and very high strength. TCL is also an effective water scavenger, used to stabilize isocyanates and urethane prepolymers.
Preparation
Terephthalic acid dichloride is produced commercially by the reaction of 1,4-bis(trichloromethyl)benzene with terephthalic acid:[2]
- C6H4(CCl3)2 + C6H4(CO2H)2 → 2 C6H4(COCl)2 + 2 HCl
It can also be obtained by chlorination of dimethyl terephthalate.[3]
Use
TCL is used for making various copolymers and aramid polymers such as Heracron, Twaron and Kevlar:
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 797. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Pfoertner, Karl-Heinz (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_573.
- ↑ "Verfahren zur Herstellung von Terephthalsäure- und Isophthalsäuredichlorid" EP patent 0095698, published 1983-12-07
External links
 |