Biology:2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase
| 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.2.99.20 | ||||||||
| CAS number | 122007-88-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
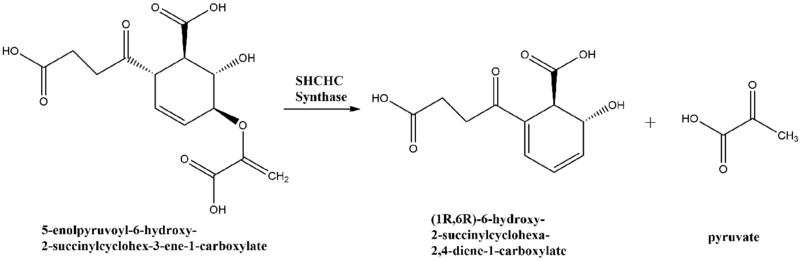
2-Succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase (EC 4.2.99.20), also known as SHCHC synthase is encoded by the menH gene in Escherichia coli and functions in the synthesis of vitamin K.[1] The specific step in the synthetic pathway that SHCHC synthase catalyzes is the conversion of 5-enolpyruvoyl-6-hydroxy-2-succinylcyclohex-3-ene-1-carboxylate to (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate and pyruvate.[2]
Background
Vitamin K is a fat soluble vitamin known to aid in blood clotting. It is recommended that all newborns receive an injection of vitamin K in order to prevent excessive bleeding of the brain after birth. There are two major forms of vitamin K that occur naturally. Phylloquinone, also known as K1, is synthesized by plants and is the major form of vitamin K in the diet. Menaquinone, K2, includes a range of forms that are synthesized by bacteria in the gut.[3]
Vitamin K is synthesized from the molecule chorismate in a nine step conversion process. SHCHC synthase catalyzes the third step in the process.[4]
Chemistry
Reaction scheme
Enzyme Structure
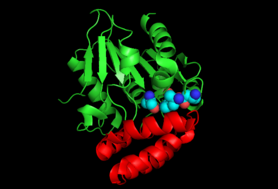
The crystal structure of the MenH enzyme in E.coli (SHCHC synthase) exists as a complex of three protein molecules shown in the diagram. SHCHC synthase forms an alpha/beta hydrolase fold with a central set of seven parallel beta sheets surrounded by alpha helixes on both sides. A cap of five alpha helixes serves to enclose the active site.[5] The enzyme exists in an open form until it binds the substrate, when it morphs into a closed form with an active catalytic triad.[6]
Energetic analysis shows that SHCHC synthase has a low energetic burden for catalytic activity.[1] This means the enzyme is more prone to mutation and is one of the most diverse enzymes in the vitamin K synthetic pathway.[7] Only fifteen amino acid residues are absolutely conserved across mutations of the enzyme.[7]
Catalytic Mechanism
The active site contains a catalytic triad of syrine, histine and arginine, which is conserved across all mutants and is proposed to initiate the reaction.[1] The triad residues are located at Ser86, Asp210, and His232.[5] This triad is proposed to catalyze a proton extraction which triggers a transfer of electrons leading to the elimination of pyruvate and formation of SHCHC.[6] Originally, it was proposed that the transition state was stabilized by a nontraditional oxyanion hole. Now a traditional oxyanion hole is favored, but not definitive.[5]
Reaction Mechanism
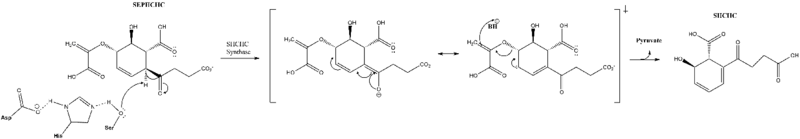
Cofactors and Alternate Reactions
SHCHC synthase is unaffected by traditional cofactors such as divalent metal ions and EDTA.[1] The enzyme is fairly specific and only acts on SEPHCHC and close derivatives.[2]
Controversy
MenH (SHCHC synthase) was previously thought to be a thioesterase involved in hydrolyzing DHNA-CoA in a later step of menaquinone synthesis. In 2008, it was determined that MenH has poor catalytic activity toward palmitoyl-CoA, casting doubt on its role as a thioesterase.[1] Direct analysis confirmed that MenH is unable to hydrolyze DHNA-CoA.[1] In 2009, it was proposed that a dedicated hotdog fold thioesterase would be needed to catalyze the hydrolysis of DHNA-CoA.[8] A protein was identified in 2013 that could fit this role.[9]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Identification and characterization of (1R,6R)-2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase in the menaquinone biosynthesis of Escherichia coli". Biochemistry 47 (11): 3426–34. March 2008. doi:10.1021/bi7023755. PMID 18284213.
- ↑ 2.0 2.1 "Information on EC 4.2.99.20 - 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase". TU Braunschweig. 2014-07-01. http://www.brenda-enzymes.org/enzyme.php?ecno=4.2.99.20.
- ↑ "Micronutrient Information Center". Linus Pauling Institute. 2014-11-30. http://lpi.oregonstate.edu/infocenter/vitamins/vitaminK/.
- ↑ van Oostende C, Widhalm JR, Furt F, Ducluzeau AL, Basset GJC (2011) Phylloquinone (Vitamin K1): function, enzymes and genes. in Advances in Botanical Research, eds Fabrice Rébeillé and Roland Douce, 59: 229-61, Academic Press (Amsterdam).
- ↑ 5.0 5.1 5.2 5.3 PDB: 4GDM; "Crystal structures of E. coli native MenH and two active site mutants". PLOS ONE 8 (4): e61325. 2013-04-18. doi:10.1371/journal.pone.0061325. PMID 23637813. Bibcode: 2013PLoSO...861325J.
- ↑ 6.0 6.1 "Molecular basis of the general base catalysis of an α/β-hydrolase catalytic triad". The Journal of Biological Chemistry 289 (22): 15867–79. May 2014. doi:10.1074/jbc.M113.535641. PMID 24737327.
- ↑ 7.0 7.1 "Catalytic mechanism of SHCHC synthase in the menaquinone biosynthesis of Escherichia coli: identification and mutational analysis of the active site residues". Biochemistry 48 (29): 6921–31. July 2009. doi:10.1021/bi900897h. PMID 19545176.
- ↑ "A dedicated thioesterase of the Hotdog-fold family is required for the biosynthesis of the naphthoquinone ring of vitamin K1". Proceedings of the National Academy of Sciences of the United States of America 106 (14): 5599–603. April 2009. doi:10.1073/pnas.0900738106. PMID 19321747. PMC 2660889. Bibcode: 2009PNAS..106.5599W. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1033&context=plantscifacpub.
- ↑ "Identification of a hotdog fold thioesterase involved in the biosynthesis of menaquinone in Escherichia coli". Journal of Bacteriology 195 (12): 2768–75. June 2013. doi:10.1128/JB.00141-13. PMID 23564174.
 |

