Biology:Aldolase B
 Generic protein structure example |
Aldolase B also known as fructose-bisphosphate aldolase B or liver-type aldolase is one of three isoenzymes (A, B, and C) of the class I fructose 1,6-bisphosphate aldolase enzyme (EC 4.1.2.13), and plays a key role in both glycolysis and gluconeogenesis. The generic fructose 1,6-bisphosphate aldolase enzyme catalyzes the reversible cleavage of fructose 1,6-bisphosphate (FBP) into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP) as well as the reversible cleavage of fructose 1-phosphate (F1P) into glyceraldehyde and dihydroxyacetone phosphate. In mammals, aldolase B is preferentially expressed in the liver, while aldolase A is expressed in muscle and erythrocytes and aldolase C is expressed in the brain. Slight differences in isozyme structure result in different activities for the two substrate molecules: FBP and fructose 1-phosphate. Aldolase B exhibits no preference and thus catalyzes both reactions, while aldolases A and C prefer FBP.[1]
In humans, aldolase B is encoded by the ALDOB gene located on chromosome 9. The gene is 14,500 base pairs long and contains 9 exons.[2][3][4] Defects in this gene have been identified as the cause of hereditary fructose intolerance (HFI).[5]
Mechanism
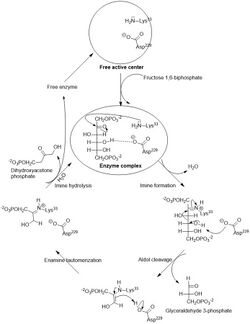
The generic fructose bisphosphate aldolase enzyme cleaves a 6-carbon fructose sugar into two 3-carbon products in a reverse aldol reaction. This reaction is typified by the formation of a Schiff base intermediate with a lysine residue (lysine 229) in the active site of the enzyme; the formation of a Schiff base is the key differentiator between Class I (produced by animals) and Class II (produced by fungi and bacteria) aldolases. After Schiff base formation, the fourth hydroxyl group on the fructose backbone is then deprotonated by an aspartate residue (aspartate 33), which results in an aldol cleavage. Schiff base hydrolysis yields two 3-carbon products. Depending on the reactant, F1P or FBP, the products are DHAP and glyceraldehyde or glyceraldehyde 3-phosphate, respectively.[6]
The ΔG°’ of this reaction is +23.9 kJ/mol. Though the reaction may seem too uphill to occur, it is of note that under physiological conditions, the ΔG of the reaction falls to close to or below zero. For example, the ΔG of this reaction under physiological conditions in erythrocytes is -0.23 kJ/mol.[6]
Structure
Aldolase B is a homotetrameric enzyme, composed of four subunits with molecular weights of 36 kDa with local 222 symmetry. Each subunit has a molecular weight of 36 kDa and contains an eight-stranded α/β barrel, which encloses lysine 229 (the Schiff-base forming amino acid that is key for catalysis).[7][8]
Isozyme specific regions
Though the majority of the overall structure of the aldolase enzyme is conserved amongst the three isozymes, four regions of the generic aldolase enzyme have been identified to be highly variable among isozymes. Such regions have been denoted isozyme-specific regions (ISR1-4). These regions are thought to give isozymes their specificities and structural differences. ISRs 1-3 are all found in exon 3 of the ALDOB gene. ISR 4 is the most variable of the four and is found at the c-terminal end of the protein.[1]
ISRs 1-3 are found predominantly in patches on the surface of the enzyme. These patches do not overlap with the active site, indicating that ISRs may change specific isozyme substrate specificity from a distance or cause the C-terminus interactions with the active site.[8] A recent theory suggests that ISRs may allow for different conformational dynamics in the aldolase enzyme that account for its specificity.[9]
Physiology
Aldolase B plays a key role in carbohydrate metabolism as it catalyzes one of the major steps of the glycolytic-gluconeogenic pathway. Though it does catalyze the breakdown of glucose, it plays a particularly important role in fructose metabolism, which occurs mostly in the liver, renal cortex, and small intestinal mucosa. When fructose is absorbed, it is phosphorylated by fructokinase to form fructose 1-phosphate. Aldolase B then catalyzes F1P breakdown into glyceraldehyde and DHAP. After glyceraldehyde is phosphorylated by triose kinase to form G3P, both products can be used in the glycolytic-gluconeogenic pathway, that is, they can be modified to become either glucose or pyruvate.[10]
Though the mechanism aldolase B regulation is unknown, increased ALDOB gene transcription in animal livers has been noticed with an increase in dietary carbohydrates and decrease in glucagon concentration.[11][12]
Interactive pathway map
Pathology
Genetic mutations leading to defects in aldolase B result in a condition called hereditary fructose intolerance. Due to the lack of functional aldolase B, organisms with HFI cannot properly process F1P, which leads to an accumulation of F1P in bodily tissues. In addition to being toxic to cellular tissues, high levels of F1P traps phosphate in an unusable form that does not return to the general phosphate pool, resulting in depletion of both phosphate and ATP stores. The lack of readily available phosphate causes the cessation of glycogenolysis in the liver, which results in hypoglycemia.[13] This accumulation also inhibits gluconeogenesis, further reducing the amount of readily available glucose. The loss of ATP leads to a multitude of problems including inhibition of protein synthesis and hepatic and renal dysfunction. Patient prognosis, however, is good in cases of hereditary fructose intolerance. By avoiding foods containing fructose, sucrose, and sorbitol, patients can live symptom-free lives.[10]
HFI is recessively inherited autosomal disorder. Approximately 30 mutations that cause HFI have been identified, and these combined mutations result in a HFI frequency of 1 in every 20,000 births.[10][14] Mutant alleles are a result of a number different types of mutations including base pair substitutions and small deletions. The most common mutation is A149P, which is a guanine to cytosine transversion in exon 5, resulting in the replacement of alanine at position 149 with proline. This specific mutant allele is estimated to account for 53% of HFI alleles.[15] Other mutations resulting in HFI are less frequent and often correlated with ancestral origins.[16]
References
- ↑ 1.0 1.1 "The structure of human liver fructose-1,6-bisphosphate aldolase". Acta Crystallogr. D 57 (Pt 11): 1526–33. November 2001. doi:10.1107/S0907444901012719. PMID 11679716.
- ↑ "Entrez Gene: ALDOB aldolase B, fructose-bisphosphate". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=229.
- ↑ "The structural gene for aldolase B (ALDB) maps to 9q13----32". Ann. Hum. Genet. 49 (Pt 3): 173–80. July 1985. doi:10.1111/j.1469-1809.1985.tb01691.x. PMID 3000275.
- ↑ "Characterization of the human aldolase B gene". Mol. Biol. Med. 3 (3): 245–64. June 1986. PMID 3016456.
- ↑ Cox TM (January 1994). "Aldolase B and fructose intolerance". FASEB J. 8 (1): 62–71. doi:10.1096/fasebj.8.1.8299892. PMID 8299892.
- ↑ 6.0 6.1 Biochemistry (4th ed.). Brooks/Cole. 2010.
- ↑ "Molecular architecture of rabbit skeletal muscle aldolase at 2.7-A resolution". Proc. Natl. Acad. Sci. U.S.A. 84 (22): 7846–50. November 1987. doi:10.1073/pnas.84.22.7846. PMID 3479768.
- ↑ 8.0 8.1 "Spatial clustering of isozyme-specific residues reveals unlikely determinants of isozyme specificity in fructose-1,6-bisphosphate aldolase". J. Biol. Chem. 278 (19): 17307–13. May 2003. doi:10.1074/jbc.M209185200. PMID 12611890.
- ↑ "Thermodynamic Analysis Shows Conformational Coupling/Dynamics Confers Substrate Specificity in Fructose-1,6-bisphosphate Aldolase". Biochemistry 46 (45): 13010–8. November 2007. doi:10.1021/bi700713s. PMID 17935305.
- ↑ 10.0 10.1 10.2 Inborn Metabolic Diseases (Fourth Revised ed.). Springer Berlin Heidelberg. 2006.
- ↑ "Dietary and hormonal regulation of aldolase B gene transcription in rat liver". Arch Biochem Biophys 314 (2): 307–14. November 1994. doi:10.1006/abbi.1994.1447. PMID 7979370.
- ↑ "Dietary and hormonal regulation of aldolase B gene expression". J. Clin. Invest. 75 (3): 1045–52. March 1985. doi:10.1172/JCI111766. PMID 2984252.
- ↑ "The biochemical basis of hereditary fructose intolerance". J. Inherit. Metab. Dis. 33 (2): 105–12. April 2010. doi:10.1007/s10545-010-9053-2. PMID 20162364.
- ↑ "Structural and functional analysis of aldolase B mutants related to hereditary fructose intolerance". FEBS Lett. 531 (2): 152–6. November 2002. doi:10.1016/S0014-5793(02)03451-8. PMID 12417303.
- ↑ "Structure of the thermolabile mutant aldolase B, A149P: molecular basis of hereditary fructose intolerance". J Mol Biol 347 (1): 135–44. March 2005. doi:10.1016/j.jmb.2005.01.008. PMID 15733923.
- ↑ Tolan DR (1995). "Molecular basis of hereditary fructose intolerance: mutations and polymorphisms in the human aldolase B gene". Hum. Mutat. 6 (3): 210–8. doi:10.1002/humu.1380060303. PMID 8535439.
Further reading
- "Molecular analysis of aldolase B genes in hereditary fructose intolerance". Lancet 335 (8685): 306–9. 1990. doi:10.1016/0140-6736(90)90603-3. PMID 1967768.
- "A new aldolase B variant, N334K, is a common cause of hereditary fructose intolerance in Yugoslavia". Nucleic Acids Res. 18 (7): 1925. 1990. doi:10.1093/nar/18.7.1925. PMID 2336380.
- "Human aldolase isozyme gene: the structure of multispecies aldolase B mRNAs". Nucleic Acids Res. 13 (14): 5055–69. 1985. doi:10.1093/nar/13.14.5055. PMID 2410860.
- "Construction and expression of human aldolase A and B expression plasmids in Escherichia coli host". Biochim. Biophys. Acta 1007 (3): 334–42. 1989. doi:10.1016/0167-4781(89)90156-5. PMID 2649152.
- "Human aldolase B gene: characterization of the genomic aldolase B gene and analysis of sequences required for multiple polyadenylations". J. Biochem. 102 (5): 1043–51. 1988. doi:10.1093/oxfordjournals.jbchem.a122142. PMID 2830249.
- "Catalytic deficiency of human aldolase B in hereditary fructose intolerance caused by a common missense mutation". Cell 53 (6): 881–5. 1988. doi:10.1016/S0092-8674(88)90349-2. PMID 3383242.
- "Isolation and nucleotide sequence of a full-length cDNA coding for aldolase B from human liver". Nucleic Acids Res. 12 (19): 7401–10. 1984. doi:10.1093/nar/12.19.7401. PMID 6548561.
- "Complete amino acid sequence for human aldolase B derived from cDNA and genomic clones". Proc. Natl. Acad. Sci. U.S.A. 81 (9): 2738–42. 1984. doi:10.1073/pnas.81.9.2738. PMID 6585824. Bibcode: 1984PNAS...81.2738R.
- "Nucleotide sequence of a cDNA clone for human aldolase B". Biochem. Biophys. Res. Commun. 117 (2): 601–9. 1984. doi:10.1016/0006-291X(83)91243-3. PMID 6689266.
- "Diverse mutations in the aldolase B gene that underlie the prevalence of hereditary fructose intolerance". Am. J. Hum. Genet. 56 (4): 1002–5. 1995. PMID 7717389.
- "Identification of a novel mutation (Leu 256→Pro) in the human aldolase B gene associated with hereditary fructose intolerance". Hum. Mol. Genet. 3 (1): 203–4. 1994. doi:10.1093/hmg/3.1.203. PMID 8162030.
- "A partially active mutant aldolase B from a patient with hereditary fructose intolerance". FASEB J. 8 (1): 107–13. 1994. doi:10.1096/fasebj.8.1.8299883. PMID 8299883.
- "Mode of interactions of human aldolase isozymes with cytoskeletons". Arch. Biochem. Biophys. 344 (1): 184–93. 1997. doi:10.1006/abbi.1997.0204. PMID 9244396.
- "Screening for hereditary fructose intolerance mutations by reverse dot-blot". Mol. Cell. Probes 13 (1): 35–40. 1999. doi:10.1006/mcpr.1998.0208. PMID 10024431.
- "Functional and molecular modelling studies of two hereditary fructose intolerance-causing mutations at arginine 303 in human liver aldolase". Biochem. J. 350 Pt 3 (Pt 3): 823–8. 2001. doi:10.1042/0264-6021:3500823. PMID 10970798.
- "Starvation-induced lysosomal degradation of aldolase B requires glutamine 111 in a signal sequence for chaperone-mediated transport". J. Cell. Physiol. 187 (1): 48–58. 2001. doi:10.1002/1097-4652(2001)9999:9999<00::AID-JCP1050>3.0.CO;2-I. PMID 11241348.
External links
- Aldolase+B at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human ALDOB genome location and ALDOB gene details page in the UCSC Genome Browser.
 |





