Biology:Animal sexual behaviour

Animal sexual behaviour takes many different forms, including within the same species. Common mating or reproductively motivated systems include monogamy, polygyny, polyandry, polygamy and promiscuity. Other sexual behaviour may be reproductively motivated (e.g. sex apparently due to duress or coercion and situational sexual behaviour) or non-reproductively motivated (e.g. homosexual sexual behaviour, bisexual sexual behaviour, cross-species sex, sexual arousal from objects or places, sex with dead animals, etc.).
When animal sexual behaviour is reproductively motivated, it is often termed mating or copulation; for most non-human mammals, mating and copulation occur at oestrus (the most fertile period in the mammalian female's reproductive cycle), which increases the chances of successful impregnation.[1][2] Some animal sexual behaviour involves competition, sometimes fighting, between multiple males. Females often select males for mating only if they appear strong and able to protect themselves. The male that wins a fight may also have the chance to mate with a larger number of females and will therefore pass on his genes to their offspring.[3]
Historically, it was believed that only humans and a small number of other species performed sexual acts other than for reproduction, and that animals' sexuality was instinctive and a simple "stimulus-response" behaviour. However, in addition to homosexual behaviours, a range of species masturbate and may use objects as tools to help them do so. Sexual behaviour may be tied more strongly to the establishment and maintenance of complex social bonds across a population which support its success in non-reproductive ways. Both reproductive and non-reproductive behaviours can be related to expressions of dominance over another animal or survival within a stressful situation (such as sex due to duress or coercion).
Mating systems
In sociobiology and behavioural ecology, the term "mating system" is used to describe the ways in which animal societies are structured in relation to sexual behaviour. The mating system specifies which males mate with which females, and under what circumstances. There are four basic systems:
Monogamy
Monogamy occurs when one male and one female mate exclusively with each other. A monogamous mating system is one in which individuals form long-lasting pairs and cooperate in raising offspring. These pairs may last for a lifetime, such as in pigeons,[6] or it may occasionally change from one mating season to another, such as in emperor penguins.[7] In contrast with tournament species, these pair-bonding species have lower levels of male aggression, competition and little sexual dimorphism. Zoologists and biologists now have evidence that monogamous pairs of animals are not always sexually exclusive. Many animals that form pairs to mate and raise offspring regularly engage in sexual activities with extra-pair partners.[8][9][10][11] This includes previous examples, such as swans. Sometimes, these extra-pair sexual activities lead to offspring. Genetic tests frequently show that some of the offspring raised by a monogamous pair come from the female mating with an extra-pair male partner.[9][12][13][14] These discoveries have led biologists to adopt new ways of talking about monogamy. According to Ulrich Reichard (2003):
Social monogamy refers to a male and female's social living arrangement (e.g., shared use of a territory, behaviour indicative of a social pair, and/or proximity between a male and female) without inferring any sexual interactions or reproductive patterns. In humans, social monogamy takes the form of monogamous marriage. Sexual monogamy is defined as an exclusive sexual relationship between a female and a male based on observations of sexual interactions. Finally, the term genetic monogamy is used when DNA analyses can confirm that a female-male pair reproduce exclusively with each other. A combination of terms indicates examples where levels of relationships coincide, e.g., sociosexual and sociogenetic monogamy describe corresponding social and sexual, and social and genetic monogamous relationships, respectively.[15]
Whatever makes a pair of animals socially monogamous does not necessarily make them sexually or genetically monogamous. Social monogamy, sexual monogamy, and genetic monogamy can occur in different combinations.
Social monogamy is relatively rare in the animal kingdom. The actual incidence of social monogamy varies greatly across different branches of the evolutionary tree. Over 90% of avian species are socially monogamous.[10][16] This stands in contrast to mammals. Only 3% of mammalian species are socially monogamous, although up to 15% of primate species are.[10][16] Social monogamy has also been observed in reptiles, fish, and insects.
Sexual monogamy is also rare among animals. Many socially monogamous species engage in extra-pair copulations, making them sexually non-monogamous. For example, while over 90% of birds are socially monogamous, "on average, 30% or more of the baby birds in any nest [are] sired by someone other than the resident male."[17] Patricia Adair Gowaty has estimated that, out of 180 different species of socially monogamous songbirds, only 10% are sexually monogamous.[18]
The incidence of genetic monogamy, determined by DNA fingerprinting, varies widely across species. For a few rare species, the incidence of genetic monogamy is 100%, with all offspring genetically related to the socially monogamous pair. But genetic monogamy is strikingly low in other species. Barash and Lipton note:
The highest known frequency of extra-pair copulations are found among the fairy-wrens, lovely tropical creatures technically known as Malurus splendens and Malurus cyaneus. More than 65% of all fairy-wren chicks are fathered by males outside the supposed breeding group.[16]p. 12
Such low levels of genetic monogamy have surprised biologists and zoologists, forcing them to rethink the role of social monogamy in evolution. They can no longer assume social monogamy determines how genes are distributed in a species. The lower the rates of genetic monogamy among socially monogamous pairs, the less of a role social monogamy plays in determining how genes are distributed among offspring.
Polygamy
The term polygamy is an umbrella term used to refer generally to non-monogamous matings. As such, polygamous relationships can be polygynous, polyandrous or polygynandrous. In a small number of species, individuals can display either polygamous or monogamous behaviour depending on environmental conditions. An example is the social wasp Apoica flavissima.[citation needed] In some species, polygyny and polyandry is displayed by both sexes in the population. Polygamy in both sexes has been observed in red flour beetle (Tribolium castaneum). Polygamy is also seen in many Lepidoptera species including Mythimna unipuncta (true armyworm moth).[19]
A tournament species is one in which "mating tends to be highly polygamous and involves high levels of male-male aggression and competition."[20] Tournament behaviour often correlates with high levels of sexual dimorphism, examples of species including chimpanzees and baboons. Most polygamous species present high levels of tournament behaviour, with a notable exception being bonobos.[citation needed]
Polygyny
Polygyny occurs when one male gets exclusive mating rights with multiple females. In some species, notably those with harem-like structures, only one of a few males in a group of females will mate. Technically, polygyny in sociobiology and zoology is defined as a system in which a male has a relationship with more than one female, but the females are predominantly bonded to a single male. Should the active male be driven out, killed, or otherwise removed from the group, in a number of species the new male will ensure that breeding resources are not wasted on another male's young.[21] The new male may achieve this in many different ways, including:
- competitive infanticide: in lions, hippopotamuses, and some monkeys, the new male will kill the offspring of the previous alpha male to cause their mothers to become receptive to his sexual advances since they are no longer nursing. To prevent this, many female primates exhibit ovulation cues among all males, and show situation-dependent receptivity.[22]
- harassment to miscarriage: amongst wild horses and baboons, the male will continually attack pregnant females until they miscarry.
- Pheromone-based spontaneous abortion
- in some rodents such as mice, a new male with a different scent will cause females who are pregnant to spontaneously fail to implant recently fertilised eggs. This does not require contact; it is mediated by scent alone. It is known as the Bruce effect.
Von Haartman specifically described the mating behaviour of the European pied flycatcher as successive polygyny.[23] Within this system, the males leave their home territory once their primary female lays her first egg. Males then create a second territory, presumably in order to attract a secondary female to breed. Even when they succeed at acquiring a second mate, the males typically return to the first female to exclusively provide for her and her offspring.[24]
Polygynous mating structures are estimated to occur in up to 90% of mammal species.[25] As polygyny is the most common form of polygamy among vertebrates (including humans), it has been studied far more extensively than polyandry or polygynandry.
Polyandry
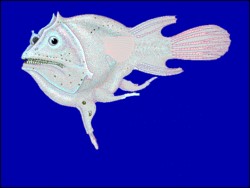
Polyandry occurs when one female gets exclusive mating rights with multiple males. In some species, such as redlip blennies, both polygyny and polyandry are observed.[26]
The males in some deep sea anglerfishes are much smaller than the females. When they find a female they bite into her skin, releasing an enzyme that digests the skin of their mouths and her body and fusing the pair down to the blood-vessel level. The male then slowly atrophies, losing first his digestive organs, then his brain, heart, and eyes, ending as nothing more than a pair of gonads, which release sperm in response to hormones in the female's bloodstream indicating egg release. This extreme sexual dimorphism ensures that, when the female is ready to spawn, she has a mate immediately available.[27] A single anglerfish female can "mate" with many males in this manner.
Polygynandry
Polygynandry occurs when multiple males mate indiscriminately with multiple females. The numbers of males and females need not be equal, and in vertebrate species studied so far, there are usually fewer males. Two examples of systems in primates are promiscuous mating chimpanzees and bonobos. These species live in social groups consisting of several males and several females. Each female copulates with many males, and vice versa. In bonobos, the amount of promiscuity is particularly striking because bonobos use sex to alleviate social conflict as well as to reproduce.[28] This mutual promiscuity is the approach most commonly used by spawning animals, and is perhaps the "original fish mating system."[4]:161 Common examples are forage fish, such as herrings, which form huge mating shoals in shallow water. The water becomes milky with sperm and the bottom is draped with millions of fertilised eggs.[4]:161
Parental investment and reproductive success

Female and male sexual behaviour differ in many species. Often, males are more active in initiating mating, and bear the more conspicuous sexual ornamentation like antlers and colourful plumage. This is a result of anisogamy, where sperm are smaller and much less costly (energetically) to produce than eggs. This difference in physiological cost means that males are more limited by the number of mates they can secure, while females are limited by the quality of genes of her mates, a phenomenon known as Bateman's principle.[29] Many females also have extra reproductive burdens in that parental care often falls mainly, or exclusively, on them. Thus, females are more limited in their potential reproductive success.[30] In species where males take on more of the reproductive costs, such as sea horses and jacanas, the role is reversed, and the females are larger, more aggressive and more brightly coloured than the males.
In hermaphroditic animals, the costs of parental care can be evenly distributed between the sexes, e.g. earthworms. In some species of planarians, sexual behaviour takes the form of penis fencing. In this form of copulation, the individual that first penetrates the other with the penis, forces the other to be female, thus carrying the majority of the cost of reproduction.[31] Post mating, banana slugs will some times gnaw off their partners penis as an act of sperm competition called apophallation.[32] This is costly as they must heal, and spend more energy courting conspecifics that can act as male and female. A hypothesis suggests these slugs may be able to compensate the loss of the male function by directing energy that would have been put towards it to the female function.[33] In the grey slug, the sharing of cost leads to a spectacular display, where the mates suspend themselves high above the ground from a slime thread, ensuring none of them can refrain from taking on the cost of egg-bearer.[34]
Seasonality

Many animal species have specific mating (or breeding) periods e.g. (seasonal breeding) so that offspring are born or hatch at an optimal time. In marine species with limited mobility and external fertilisation like corals, sea urchins and clams, the timing of the common spawning is the only externally visible form of sexual behaviour. In areas with continuously high primary production, some species have a series of breeding seasons throughout the year. This is the case with most primates (who are primarily tropical and subtropical animals). Some animals (opportunistic breeders) breed dependent upon other conditions in their environment aside from time of year.
Mammals
Mating seasons are often associated with changes to herd or group structure, and behavioural changes, including territorialism amongst individuals. These may be annual (e.g. wolves), biannual (e.g. dogs) or more frequently (e.g. horses). During these periods, females of most mammalian species are more mentally and physically receptive to sexual advances, a period scientifically described as oestrus but commonly described as being "in season" or "in heat". Sexual behaviour may occur outside oestrus,[35] and such acts as do occur are not necessarily harmful.[36]
Some mammals (e.g. domestic cats, rabbits and camelids) are termed "induced ovulators". For these species, the female ovulates due to an external stimulus during, or just prior to, mating, rather than ovulating cyclically or spontaneously. Stimuli causing induced ovulation include the sexual behaviour of coitus, sperm and pheromones. Domestic cats have penile spines. Upon withdrawal of a cat's penis, the spines rake the walls of the female's vagina, which may cause ovulation.[37][38]
Amphibians
For many amphibians, an annual breeding cycle applies, typically regulated by ambient temperature, precipitation, availability of surface water and food supply. This breeding season is accentuated in temperate regions, in boreal climate the breeding season is typically concentrated to a few short days in the spring. Some species, such as the Rana clamitans (green frog), spend from June to August defending their territory. In order to protect these territories, they use five vocalizations.[39]
Fish
Like many coral reef dwellers, the clownfish spawn around the time of the full moon in the wild. In a group of clownfish, there is a strict dominance hierarchy. The largest and most aggressive female is found at the top. Only two clownfish, a male and a female, in a group reproduce through external fertilisation. Clownfish are sequential hermaphrodites, meaning that they develop into males first, and when they mature, they become females. If the female clownfish is removed from the group, such as by death, one of the largest and most dominant males will become a female. The remaining males will move up a rank in the hierarchy.
Motivation
Various neurohormones stimulate sexual wanting in animals. In general, studies have suggested that dopamine is involved in sexual incentive motivation, oxytocin and melanocortins in sexual attraction, and noradrenaline in sexual arousal.[40] Vasopressin is also involved in the sexual behaviour of some animals.[41]
Neurohormones in the mating systems of voles
The mating system of prairie voles is monogamous; after mating, they form a lifelong bond. In contrast, montane voles have a polygamous mating system. When montane voles mate, they form no strong attachments, and separate after copulation. Studies[42] on the brains of these two species have found that it is two neurohormones and their respective receptors that are responsible for these differences in mating strategies. Male prairie voles release vasopressin after copulation with a partner, and an attachment to their partner then develops. Female prairie voles release oxytocin after copulation with a partner, and similarly develop an attachment to their partner.
Neither male nor female montane voles release high quantities of oxytocin or vasopressin when they mate. Even when injected with these neurohormones, their mating system does not change. In contrast, if prairie voles are injected with the neurohormones, they may form a lifelong attachment, even if they have not mated. The differing response to the neurohormones between the two species is due to a difference in the number of oxytocin and vasopressin receptors. Prairie voles have a greater number of oxytocin and vasopressin receptors compared to montane voles, and are therefore more sensitive to those two neurohormones. It's believed that it's the quantity of receptors, rather than the quantity of the hormones, that determines the mating system and bond-formation of either species.[citation needed]
Oxytocin and rat sexual behaviour
Mother rats experience a postpartum estrus which makes them highly motivated to mate. However, they also have a strong motivation to protect their newly born pups. As a consequence, the mother rat solicits males to the nest but simultaneously becomes aggressive towards them to protect her young. If the mother rat is given injections of an oxytocin receptor antagonist, they no longer experience these maternal motivations.[43]
Prolactin influences social bonding in rats.[43]
Oxytocin and primate sexual behaviour
Oxytocin plays a similar role in non-human primates as it does in humans.
Grooming, sex, and cuddling frequencies correlate positively with levels of oxytocin. As the level of oxytocin increases so does sexual motivation. While oxytocin plays a major role in parent child relationships, it is also found to play a role in adult sexual relationships. Its secretion affects the nature of the relationship or if there will even be a relationship at all.[citation needed][44]
Studies have shown that oxytocin is higher in monkeys in lifelong monogamous relationships compared to monkeys which are single. Furthermore, the oxytocin levels of the couples correlate positively; when the oxytocin secretion of one increases, the other one also increases. Higher levels of oxytocin are related to monkeys expressing more behaviours such as cuddling, grooming and sex, while lower levels of oxytocin reduce motivation for these activities.[citation needed]
Research on oxytocin's role in the animal brain suggests that it plays less of a role in behaviours of love and affection than previously believed. "When oxytocin was first discovered in 1909, it was thought mostly to influence a mother's labour contractions and milk let-down. Then, in the 1990s, research with prairie voles found that giving them a dose of oxytocin resulted in the formation of a bond with their future mate (Azar, 40)." Oxytocin has since been treated by the media as the sole player in the "love and mating game" in mammals. This view, however, is proving to be false as, "most hormones don't influence behaviour directly. Rather, they affect thinking and emotions in variable ways (Azar, 40)." There is much more involved in sexual behaviour in the mammalian animal than oxytocin and vasopressin can explain.[45][46][47][48][49][50][51][52][53][54]
Pleasure
It is often assumed that animals do not have sex for pleasure, or alternatively that humans, pigs, bonobos (and perhaps dolphins and one or two more species of primates) are the only species that do. This is sometimes stated as "animals mate only for reproduction". This view is considered a misconception by some scholars.[55][56] Jonathan Balcombe argues that the prevalence of non-reproductive sexual behaviour in certain species suggests that sexual stimulation is pleasurable. He also points to the presence of the clitoris in some female mammals, and evidence for female orgasm in primates.[57] On the other hand, it is impossible to know the subjective feelings of animals,[40] and the notion that non-human animals experience emotions similar to humans is a contentious subject.[58][59][60][61]
A 2006 Denmark Animal Ethics Council report,[62] which examined current knowledge of animal sexuality in the context of legal queries concerning sexual acts by humans, has the following comments, primarily related to domestically common animals:
Even though the evolution-related purpose of mating can be said to be reproduction, it is not actually the creating of offspring which originally causes them to mate. It is probable that they mate because they are motivated for the actual copulation, and because this is connected with a positive experience. It is therefore reasonable to assume that there is some form of pleasure or satisfaction connected with the act. This assumption is confirmed by the behaviour of males, who in the case of many species are prepared to work to get access to female animals, especially if the female animal is in oestrus, and males who for breeding purposes are used to having sperm collected become very eager, when the equipment they associate with the collection is taken out. . . . There is nothing in female mammals' anatomy or physiology that contradicts that stimulation of the sexual organs and mating is able to be a positive experience. For instance, the clitoris acts in the same way as with women, and scientific studies have shown that the success of reproduction is improved by stimulation of clitoris on (among other species) cows and mares in connection with insemination, because it improves the transportation of the sperm due to contractions of the inner genitalia. This probably also applies to female animals of other animal species, and contractions in the inner genitals are seen e.g. also during orgasm for women. It is therefore reasonable to assume that sexual intercourse may be linked with a positive experience for female animals.
Koinophilia
Koinophilia is the love of the "normal" or phenotypically common (from the Greek κοινός, koinós, meaning "usual" or "common").[63] The term was introduced to scientific literature in 1990, and refers to the tendency of animals seeking a mate to prefer that mate not to have any unusual, peculiar or deviant features.[63] Similarly, animals preferentially choose mates with low fluctuating asymmetry.[64] However, animal sexual ornaments can evolve through runaway selection, which is driven by (usually female) selection for non-standard traits.[65]
Interpretation bias
The field of study of sexuality in non-human species was a long-standing taboo.[66][unreliable source?] In the past, researchers sometimes failed to observe, miscategorised or misdescribed sexual behaviour which did not meet their preconceptions—their bias tended to support what would now be described as conservative sexual mores. An example of overlooking behaviour relates to descriptions of giraffe mating:
When nine out of ten pairings occur between males, "[e]very male that sniffed a female was reported as sex, while anal intercourse with orgasm between males was only [categorized as] 'revolving around' dominance, competition or greetings."[66]
In the 21st century, liberal social or sexual views are often projected upon animal subjects of research. Popular discussions of bonobos are a frequently cited example. Current research frequently expresses views such as that of the Natural History Museum at the University of Oslo, which in 2006 held an exhibition on animal sexuality:
Many researchers have described homosexuality as something altogether different from sex. They must realise that animals can have sex with who they will, when they will and without consideration to a researcher's ethical principles.[66]
Other animal activities may be misinterpreted due to the frequency and context in which animals perform the behaviour. For example, domestic ruminants display behaviours such as mounting and head-butting. This often occurs when the animals are establishing dominance relationships and are not necessarily sexually motivated. Careful analysis must be made to interpret what animal motivations are being expressed by those behaviours.[67]
Types of sexual behaviour
Reproductive sexual behaviour
Copulation
Copulation is the union of the male and female sex organs, the sexual activity specifically organized to transmit male sperm into the body of the female.[68]
Cuckoldry

Alternative male strategies which allow small males to engage in cuckoldry can develop in species such as fish where spawning is dominated by large and aggressive males. Cuckoldry is a variant of polyandry, and can occur with sneak spawners. A sneak spawner is a male that rushes in to join the spawning rush of a spawning pair.[69] A spawning rush occurs when a fish makes a burst of speed, usually on a near vertical incline, releasing gametes at the apex, followed by a rapid return to the lake or sea floor or fish aggregation.[70] Sneaking males do not take part in courtship. In salmon and trout, for example, jack males are common. These are small silvery males that migrate upstream along with the standard, large, hook-nosed males and that spawn by sneaking into redds to release sperm simultaneously with a mated pair. This behaviour is an evolutionarily stable strategy for reproduction, because it is favoured by natural selection just like the "standard" strategy of large males.[71]
Hermaphroditism
Hermaphroditism occurs when a given individual in a species possesses both male and female reproductive organs, or can alternate between possessing first one, and then the other. Hermaphroditism is common in invertebrates but rare in vertebrates. It can be contrasted with gonochorism, where each individual in a species is either male or female, and remains that way throughout their lives. Most fish are gonochorists, but hermaphroditism is known to occur in 14 families of teleost fishes.[72]
Usually hermaphrodites are sequential, meaning they can switch sex, usually from female to male (protogyny). This can happen if a dominant male is removed from a group of females. The largest female in the harem can switch sex over a few days and replace the dominant male.[72] This is found amongst coral reef fishes such as groupers, parrotfishes and wrasses. As an example, most wrasses are protogynous hermaphrodites within a haremic mating system.[73][74] It is less common for a male to switch to a female (protandry).[4]:162 A common example of a protandrous species are clownfish—if the larger, dominant female dies, in many cases, the reproductive male gains weight and becomes the female.[75][76] Hermaphroditism allows for complex mating systems. Wrasses exhibit three different mating systems: polygynous, lek-like, and promiscuous mating systems.[77]
Sexual cannibalism
File:Araneus diadematus - mating behaviour - short.ogv
Sexual cannibalism is a behaviour in which a female animal kills and consumes the male before, during, or after copulation. Sexual cannibalism confers fitness advantages to both the male and female.[78] Sexual cannibalism is common among insects, arachnids[79] and amphipods.[79] There is also evidence of sexual cannibalism in gastropods and copepods.[80]
Sexual coercion

Sex in a forceful or apparently coercive context has been documented in a variety of species. In some herbivorous herd species, or species where males and females are very different in size, the male dominates sexually by force and size.[citation needed]
Some species of birds have been observed combining sexual intercourse with apparent violent assault; these include ducks,[81][82] and geese.[83] Female white-fronted bee-eaters are subjected to forced copulations. When females emerge from their nest burrows, males sometimes force them to the ground and mate with them. Such forced copulations are made preferentially on females who are laying and who may therefore lay eggs fertilized by the male.[84]
It has been reported that young male elephants in South Africa sexually coerced and killed rhinoceroses.[85] This interpretation of the elephants' behaviour was disputed by one of the original study's authors, who said there was "nothing sexual about these attacks".[86]
Parthenogenesis
Parthenogenesis is a form of asexual reproduction in which growth and development of embryos occur without fertilisation.[87] Technically, parthenogenesis is not a behaviour, however, sexual behaviours may be involved.
Whip-tailed lizard females have the ability to reproduce through parthenogenesis and as such males are rare and sexual breeding non-standard. Females engage in "pseudocopulation"[88] to stimulate ovulation, with their behaviour following their hormonal cycles; during low levels of oestrogen, these (female) lizards engage in "masculine" sexual roles. Those animals with currently high oestrogen levels assume "feminine" sexual roles. Lizards that perform the courtship ritual have greater fecundity than those kept in isolation due to an increase in hormones triggered by the sexual behaviours. So, even though asexual whiptail lizards populations lack males, sexual stimuli still increase reproductive success. From an evolutionary standpoint these females are passing their full genetic code to all of their offspring rather than the 50% of genes that would be passed in sexual reproduction.[citation needed]
It is rare to find true parthenogenesis in fishes, where females produce female offspring with no input from males. All-female species include the Texas silverside, Menidia clarkhubbsi[89] and a complex of Mexican mollies.[4]:162
Parthenogenesis has been recorded in 70 vertebrate species[90] including hammerhead sharks,[91] blacktip sharks,[92] amphibians[93] and lizards.[94]
Unisexuality
Unisexuality occurs when a species is all-male or all-female. Unisexuality occurs in some fish species and can take complex forms. Squalius alburnoides, a minnow found in several river basins in Portugal and Spain, appears to be an all-male species. The existence of this species illustrates the potential complexity of mating systems in fish. The species originated as a hybrid between two species and is diploid but not hermaphroditic. It can have triploid and tetraploid forms, including all-female forms that reproduce mainly through hybridogenesis.[95]
Others
- Interbreeding: Hybrid offspring can result from the mating of two organisms of distinct but closely related parent species, although the resulting offspring is not always fertile. According to Alfred Kinsey, genetic studies on wild animal populations have shown a "large number" of inter-species hybrids.[96]
- Prostitution: There are reports that animals occasionally engage in prostitution. A small number of pair-bonded females within a group of penguins took nesting material (stones) after copulating with a non-partner male. The researcher stated "I was watching opportunistically, so I can't give an exact figure of how common it really is."[97] It has been reported that "bartering of meat for sex ... forms part of the social fabric of a troop of wild chimps living in the Tai National Park in the Côte d'Ivoire."[98]
- Pavlovian conditioning: The sexualisation of objects or locations is recognised in the animal breeding world. For example, male animals may become sexually aroused upon visiting a location where they have been allowed to have sex before, or upon seeing a stimulus previously associated with sexual activity such as an artificial vagina.[99] Sexual preferences for certain cues can be artificially induced in rats by pairing scents or objects with their early sexual experiences.[100] The primary motivation of this behaviour is Pavlovian conditioning, and the association is due to a conditioned response (or association) formed with a distinctive "reward".[100]
- Viewing images: A study using four adult male rhesus macaques (Macaca mulatta) showed that male rhesus macaques will give up a highly valued item, juice, to see images of the faces or perineum of high-status females.[101] Encouraging captive pandas to mate is problematic. Showing young male pandas "panda pornography" is credited with a recent population boom among pandas in captivity in China. One researcher attributed the success to the sounds on the recordings.[102]
- Copulatory wounding and traumatic insemination: Injury to a partner's genital tract during mating occurs in at least 40 taxa, ranging from fruit flies to humans. However, it often goes unnoticed due to its cryptic nature and because of internal wounds not visible outside.[103]
Non-reproductive sexual behaviour
There is a range of behaviours that animals perform that appear to be sexually motivated but which can not result in reproduction. These include:
- Masturbation: Some species, both male and female, masturbate, both when partners are available and otherwise.[104][105]
- Oral sex: Several species engage in both autofellatio and oral sex. This has been documented in brown bears,[106] Tibetan macaques,[107] wolves,[108] goats, primates, bats,[109][110] cape ground squirrels[111] and sheep. In the greater short-nosed fruit bat, copulation by males is dorsoventral and the females lick the shaft or the base of the male's penis, but not the glans which has already penetrated the vagina. While the females do this, the penis is not withdrawn and research has shown a positive relationship between length of the time that the penis is licked and the duration of copulation. Post copulation genital grooming has also been observed.[112]
- Homosexuality: Same-sex sexual behaviour occurs in a range of species, especially in social species, particularly in marine birds and mammals, monkeys, and the great apes. (As of 1999), the scientific literature contained reports of homosexual behaviour in at least 471 wild species.[113] Organisers of the Against Nature? exhibit stated that "homosexuality has been observed among 1,500 species, and that in 500 of those it is well documented."[114]

- Genital-genital rubbing: This is sexual activity in which one animal rubs his or her genitals against the genitals of another animal. This is stated to be the "bonobo's most typical sexual pattern, undocumented in any other primate".[116][117]
- Inter-species mating: Some animals opportunistically mate with individuals of another species.[118]
- Sex involving juveniles: Male stoats (Mustela erminea) will sometimes mate with infant females of their species.[119] This is a natural part of their reproductive biology—they have a delayed gestation period, so these females give birth the following year when they are fully grown. Juvenile male common chimpanzees have been recorded mounting and copulating with immature chimps. Infants in bonobo societies are often involved in sexual behaviour.[120]
- Necrophilia: This describes when an animal engages in a sexual act with a dead animal. It has been observed in mammals, birds, reptiles and frogs.[121]
- Bisexuality: This describes when an animal shows sexual behaviour towards both males and females.
- Extended female sexuality: This is when females mate with males outside of their conceptive period.[122][22]
Seahorse
Seahorses, once considered to be monogamous species with pairs mating for life, were described in a 2007 study as "promiscuous, flighty, and more than a little bit gay".[123] Scientists at 15 aquaria studied 90 seahorses of three species. Of 3,168 sexual encounters, 37% were same-sex acts. Flirting was common (up to 25 potential partners a day of both sexes); only one species (the British spiny seahorse) included faithful representatives, and for these 5 of 17 were faithful, 12 were not. Bisexual behaviour was widespread and considered "both a great surprise and a shock", with big-bellied seahorses of both sexes not showing partner preference. 1,986 contacts were male-female, 836 were female-female and 346 were male-male.[123]
Bonobo
Among bonobos, males and females engage in sexual behaviour with the same and the opposite sex, with females being particularly noted for engaging in sexual behaviour with each other and at up to 75% of sexual activity being non-reproductive, as being sexually active does not necessarily correlate with their ovulation cycles.[116] Sexual activity occurs between almost all ages and sexes of bonobo societies.[124][125] Primatologist Frans de Waal believes that bonobos use sexual activity to resolve conflict between individuals.[28][126] Immature bonobos, contrariwise, perform genital contact when relaxed.[125]
Macaque
Similar same-sex sexual behaviours occur in both male and female macaques.[127] It is thought to be done for pleasure as an erect male mounts and thrusts upon or into another male.[127][128] Sexual receptivity can also be indicated by red faces and shrieking.[127] Mutual ejaculation after a combination of anal intercourse and masturbation has also been witnessed, although it may be rare.[128] In comparison to socio-sexual behaviours such as dominance displays, homosexual mounts last longer, happen in series, and usually involve pelvic thrusting.[127]
Females are also thought to participate for pleasure as vulvar, perineal, and anal stimulation is part of these interactions. The stimulation can come from their own tails, mounting their partner, thrusting or a combination of these.[129]
Dolphin
Male bottlenose dolphins have been observed working in pairs to follow or restrict the movement of a female for weeks at a time, waiting for her to become sexually receptive. The same pairs have also been observed engaging in intense sexual play with each other. Janet Mann, a professor of biology and psychology at Georgetown University, argues[130] that the common same-sex behaviour among male dolphin calves is about bond formation and benefits the species evolutionarily. Studies have shown the dolphins later in life are bisexual and the male bonds forged from homosexuality work for protection as well as locating females with which to reproduce.[130]
In 1991, an English man was prosecuted for allegedly having sexual contact with a dolphin.[131] The man was found not guilty after it was revealed at trial that the dolphin was known to tow bathers through the water by hooking his penis around them.[131]
Hyena
The female spotted hyena has a unique urinary-genital system, closely resembling the penis of the male, called a pseudo-penis. Dominance relationships with strong sexual elements are routinely observed between related females. They are notable for using visible sexual arousal as a sign of submission but not dominance in males as well as females (females have a sizeable erectile clitoris).[132] It is speculated that to facilitate this, their sympathetic and parasympathetic nervous systems may be partially reversed in respect to their reproductive organs.[133]
Mating behaviour
Vertebrates
Mammals
Mammals mate by vaginal copulation. To achieve this, the male usually mounts the female from behind.[134] The female may exhibit lordosis in which she arches her back ventrally to facilitate entry of the penis. Amongst the land mammals, other than humans, only bonobos mate in a face-to-face position,[135][better source needed] as the females' anatomy seems to reflect,[116] although ventro-ventral copulation has also been observed in Rhabdomys.[136] Some sea mammals copulate in a belly-to-belly position.[137][138] Some camelids mate in a lying-down position.[139] In most mammals ejaculation occurs after multiple intromissions,[140] but in most primates, copulation consists of one brief intromission.[141] In most ruminant species, a single pelvic thrust occurs during copulation.[142][143] In most deer species, a copulatory jump also occurs.[144][145]
During mating, a "copulatory tie" occurs in mammals such as fossas,[146] canids[147] and Japanese martens.[148] A "copulatory lock" also occurs in some primate species, such as Galago senegalensis.[149]
The copulatory behaviour of many mammalian species is affected by sperm competition.[150]
Some females have concealed fertility, making it difficult for males to evaluate if a female is fertile. This is costly as ejaculation expends much energy.[22]
Invertebrates
Invertebrates are often hermaphrodites. Some hermaphroditic land snails begin mating with an elaborate tactile courting ritual. The two snails circle around each other for up to six hours, touching with their tentacles, and biting lips and the area of the genital pore, which shows some preliminary signs of the eversion of the penis. As the snails approach mating, hydraulic pressure builds up in the blood sinus surrounding an organ housing a sharpened dart. The dart is made of calcium carbonate or chitin, and is called a love dart. Each snail manoeuvres to get its genital pore in the best position, close to the other snail's body. Then, when the body of one snail touches the other snail's genital pore, it triggers the firing of the love dart.[151] After the snails have fired their darts, they copulate and exchange sperm as a separate part of the mating progression. The love darts are covered with a mucus that contains a hormone-like substance that facilitates the survival of the sperm.[152][153]
Penis fencing is a mating behaviour engaged in by certain species of flatworm, such as Pseudobiceros bedfordi. Species which engage in the practice are hermaphroditic, possessing both eggs and sperm-producing testes.[154] The species "fence" using two-headed dagger-like penises which are pointed, and white in colour. One organism inseminates the other. The sperm is absorbed through pores in the skin, causing fertilisation.
Corals can be both gonochoristic (unisexual) and hermaphroditic, each of which can reproduce sexually and asexually. Reproduction also allows corals to settle new areas. Corals predominantly reproduce sexually. 25% of hermatypic corals (stony corals) form single sex (gonochoristic) colonies, while the rest are hermaphroditic.[155] About 75% of all hermatypic corals "broadcast spawn" by releasing gametes – eggs and sperm – into the water to spread offspring. The gametes fuse during fertilisation to form a microscopic larva called a planula, typically pink and elliptical in shape.[156] Synchronous spawning is very typical on the coral reef and often, even when multiple species are present, all corals spawn on the same night. This synchrony is essential so that male and female gametes can meet. Corals must rely on environmental cues, varying from species to species, to determine the proper time to release gametes into the water. The cues involve lunar changes, sunset time, and possibly chemical signalling.[155] Synchronous spawning may form hybrids and is perhaps involved in coral speciation.[157]
Butterflies spend much time searching for mates. When the male spots a mate, he will fly closer and release pheromones. He then performs a special courtship dance to attract the female. If the female appreciates the dancing she may join him. Then they join their bodies together end to end at their abdomens. Here, the male passes the sperm to the female's egg-laying tube, which will soon be fertilised by the sperm.[158]
Many animals make plugs of mucus to seal the female's orifice after mating. Normally such plugs are secreted by the male, to block subsequent partners. In spiders the female can assist the process.[159] Spider sex is unusual in that males transfer their sperm to the female on small limbs called pedipalps. They use these to pick their sperm up from their genitals and insert it into the female's sexual orifice, rather than copulating directly.[159] On the 14 occasions a sexual plug was made, the female produced it without assistance from the male. On ten of these occasions the male's pedipalps then seemed to get stuck while he was transferring the sperm (which is rarely the case in other species of spider), and he had great difficulty freeing himself. In two of those ten instances, he was eaten as a result.[159]
In the orb-weaving spider species Zygiella x-notata, individuals engage in a variety of sexual behaviors including male choosiness, mate guarding, and vibrational signaling in courtship.[160][161]
Genetic evidence of interspecies sexual activity in humans
Research into human evolution confirms that, in some cases, interspecies sexual activity may have been responsible for the evolution of new species (speciation). Analysis of animal genes found evidence that, after humans had diverged from other apes, interspecies mating nonetheless occurred regularly enough to change certain genes in the new gene pool.[162] Researchers found that the X chromosomes of humans and chimps may have diverged around 1.2 million years after the other chromosomes. One possible explanation is that modern humans emerged from a hybrid of human and chimp populations.[163] A 2012 study questioned this explanation, concluding that "there is no strong reason to involve complicated factors in explaining the autosomal data".[164][dubious ]
Inbreeding avoidance
When close relatives mate, progeny may exhibit the detrimental effects of inbreeding depression. Inbreeding depression is predominantly caused by the homozygous expression of recessive deleterious alleles.[165] Over time, inbreeding depression may lead to the evolution of inbreeding avoidance behaviour. Several examples of animal behaviour that reduce mating of close relatives and inbreeding depression are described next.
Reproductively active female naked mole-rats tend to associate with unfamiliar males (usually non-kin), whereas reproductively inactive females do not discriminate.[166] The preference of reproductively active females for unfamiliar males is interpreted as an adaptation for avoiding inbreeding.
When mice inbreed with close relatives in their natural habitat, there is a significant detrimental effect on progeny survival.[167] In the house mouse, the major urinary protein (MUP) gene cluster provides a highly polymorphic scent signal of genetic identity that appears to underlie kin recognition and inbreeding avoidance. Thus there are fewer matings between mice sharing MUP haplotypes than would be expected if there were random mating.[168]
Meerkat females appear to be able to discriminate the odour of their kin from the odour of their non-kin.[169] Kin recognition is a useful ability that facilitates both cooperation among relatives and the avoidance of inbreeding. When mating does occur between meerkat relatives, it often results in inbreeding depression. Inbreeding depression was evident for a variety of traits: pup mass at emergence from the natal burrow, hind-foot length, growth until independence and juvenile survival.[170]
The grey-sided vole (Myodes rufocanus) exhibits male-biased dispersal as a means of avoiding incestuous matings.[171] Among those matings that do involve inbreeding the number of weaned juveniles in litters is significantly smaller than that from non-inbred litters indicating inbreeding depression.
In natural populations of the bird Parus major (great tit), inbreeding is likely avoided by dispersal of individuals from their birthplace, which reduces the chance of mating with a close relative.[172]
Toads display breeding site fidelity, as do many amphibians. Individuals that return to natal ponds to breed will likely encounter siblings as potential mates. Although incest is possible, Bufo americanus siblings rarely mate. These toads likely recognise and actively avoid close kins as mates. Advertisement vocalisations by males appear to serve as cues by which females recognise their kin.[173]
See also
- Pre-copulatory isolation mechanisms in animals
- Biology and sexual orientation
- Green Porno, a series of short films about animal mating, enacted by humans, airing on the Sundance Channel
- List of animals displaying homosexual behaviour
- r/K selection theory
- Polygamy in house mouse
- Sexual behaviour of dogs
- Sexual behaviour of horses
References
- ↑ Kent, Michael (2000). Advanced biology. Oxford University Press. pp. 250–253. ISBN 978-0-19-914195-1. https://books.google.com/books?id=8aw4ZWLABQkC&pg=PA250.
- ↑ Thorpe, Showick; Thorpe, Edgar (2009). General Studies Manual. Pearson Education India. p. 17. ISBN 9788131721339. https://books.google.com/books?id=oAo1X2eagywC&pg=RA1-PR17.
- ↑ Wickler, Wolfgang; Lorenz; Konrad; Kacher, Hermann (1974). The sexual code : the social behaviour of animals and men.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Moyle PB and Cech JJ (2004) Fishes, An Introduction to Ichthyology. 5th Ed, Benjamin Cummings. ISBN:978-0-13-100847-2
- ↑ Berglund A (1997) "Mating systems and sex allocation" Pages 237–265 in JJ Godon, ed. Behavioural ecology of teleost fishes. Oxford University Press. ISBN:0-19-850503-5.
- ↑ "Pigeon". Encyclopædia Britannica. https://www.britannica.com/EBchecked/topic/460131/pigeon. Retrieved 26 August 2014.
- ↑ Lily Whiteman (13 February 2013). "Animal Attraction: The Many Forms of Monogamy in the Animal Kingdom". National Science Foundation. https://www.nsf.gov/discoveries/disc_summ.jsp?cntn_id=126932.
- ↑ Ågren, G.; Zhou, Q.; Zhong, W. (1989). "Ecology and social behaviour of Mongolian gerbils, Meriones unguiculatus, at Xilinhot, Inner Mongolia, China". Animal Behaviour 37: 11–27. doi:10.1016/0003-3472(89)90002-X.
- ↑ Jump up to: 9.0 9.1 Birkhead, T.R.; Møller, A.P. (1995). "Extra-pair copulations and extra-pair paternity in birds". Animal Behaviour 49 (3): 843–848. doi:10.1016/0003-3472(95)80217-7.
- ↑ Jump up to: 10.0 10.1 10.2 Reichard, U.H. (2002). "Monogamy – a variable relationship". Max Planck Research 3: 62–67. http://www.mpg.de/1028786/W001_Biology-Medicine_062_067.pdf. Retrieved 24 April 2013.
- ↑ Westneat, D. F.; Stewart, I. R. K. (2003). "Extra-Pair Paternity in Birds: Causes, Correlates, and Conflict". Annual Review of Ecology, Evolution, and Systematics 34: 365–396. doi:10.1146/annurev.ecolsys.34.011802.132439.
- ↑ Birkhead, T.R. & Møller, A.P. (1996) "Monogamy and sperm competition in birds". In J. M. Black (Ed.), Partnerships in Birds: The Study of Monogamy, pp. 323–343, Oxford: Oxford University Press ISBN:0-19-854860-5.
- ↑ Owens, I. P. F.; Hartley, I. R. (1998). "Sexual dimorphism in birds: Why are there so many different forms of dimorphism?". Proceedings of the Royal Society B: Biological Sciences 265 (1394): 397–407. doi:10.1098/rspb.1998.0308.
- ↑ Solomon, N. G.; Keane, B.; Knoch, L. R.; Hogan, P. J. (2004). "Multiple paternity in socially monogamous prairie voles (Microtus ochrogaster)". Canadian Journal of Zoology 82 (10): 1667–1671. doi:10.1139/z04-142.
- ↑ Reichard, U.H. (2003). Monogamy: Past and present. In U.H. Reichard and C. Boesch (Eds.), Monogamy: Mating strategies and partnerships in birds, humans, and other mammals, pp. 3–25, Cambridge: Cambridge University Press, ISBN:0521819733.
- ↑ Jump up to: 16.0 16.1 16.2 Barash, D.P. & Lipton, J.E. (2001). The Myth of Monogamy. New York, NY: W.H. Freeman and Company, ISBN:0805071369.
- ↑ Angier, Natalie (21 August 1990). "Mating for Life? It's Not for the Birds of the Bees". The New York Times. https://query.nytimes.com/gst/fullpage.html?res=9C0CEFDC143FF932A1575BC0A966958260.
- ↑ Morell, V. (1998). "Evolution of Sex: A New Look at Monogamy". Science 281 (5385): 1982–1983. doi:10.1126/science.281.5385.1982. PMID 9767050.
- ↑ Mcneil, Jeremy N (1986). "Calling Behavior: Can It Be Used to Identify Migratory Species of Moths". The Florida Entomologist 69 (1): 78–84. doi:10.2307/3494746.
- ↑ Robert Sapolsky (2005). "Biology and Human Behavior: The Neurological Origins of Individuality, 2nd edition". The Teaching Company. http://www.thegreatcourses.com/tgc/courses/course_detail.aspx?cid=1597.
- ↑ This section and examples taken from Robert Sapolsky (1998) Why Zebras Don't Get Ulcers, W.H. Freeman and Co., ISBN:0-7167-3210-6, pp. 140–141.
- ↑ Jump up to: 22.0 22.1 22.2 Fürtbauer, Ines; Heistermann, Michael; Schülke, Oliver; Ostner, Julia (2011-08-10). "Concealed Fertility and Extended Female Sexuality in a Non-Human Primate (Macaca assamensis)". PLOS ONE 6 (8): e23105. doi:10.1371/journal.pone.0023105. ISSN 1932-6203. PMID 21853074. Bibcode: 2011PLoSO...623105F.
- ↑ Haartman, L. V. (1951). "Successive Polygamy". Behaviour 3: 256–273. doi:10.1163/156853951X00296.
- ↑ Silverin, B. (1979). "Effects of long-acting testosterone administration on testes in free living pied flycatchers Ficedula hypoleuca". Endokrinologie 74 (2): 141–146. PMID 583410.
- ↑ Aloise King, Edith D.; Banks, Peter B.; Brooks, Robert C. (2011). "Sexual conflict in mammals: consequences for mating systems and life history". Mammal Review 43 (1): 47–58. January 2013. doi:10.1111/j.1365-2907.2011.00200.x.
- ↑ Marraro, CH; Nursall JR (1983). "The reproductive periodicity and behaviour of Ophioblennius atlanticus at Barbados". J Zool 61 (2): 317–325. doi:10.1139/z83-042.
- ↑ Theodore W. Pietsch (1975). "Precocious sexual parasitism in the deep sea ceratioid anglerfish, Cryptopsaras couesi Gill". Nature 256 (5512): 38–40. doi:10.1038/256038a0. Bibcode: 1975Natur.256...38P.
- ↑ Jump up to: 28.0 28.1 "Homosexual Activity Among Animals Stirs Debate". 2004-07-23. http://news.nationalgeographic.com/news/2004/07/0722_040722_gayanimal_2.html.
- ↑ Bateman, A.J. (1948), "Intra-sexual selection in Drosophila", Heredity 2 (Pt. 3): 349–368, doi:10.1038/hdy.1948.21, PMID 18103134
- ↑ Trivers, R.L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man, 1871–1971 (pp. 136–179). Chicago, IL: Aldine. ISBN:0-435-62157-2
- ↑ Hermaphrodites duel for manhood , Science News Online. Accessed 14 March 2009.
- ↑ Miller, Brooke L. W (2007). Sexual conflict and partner manipulation in the banana slug, Ariolimax dolichophallus. Santa Cruz: University of California. http://gradworks.umi.com/32/65/3265732.html. Retrieved 13 February 2015.
- ↑ Backeljau, Thierry; Jordaens, Kurt; Dillen, Lobke (2007). "Effects of mating, breeding system and parasites on reproduction in hermaphrodites: Pulmonate gastropods (Mollusca)". Animal Biology 57 (2): 137–195. doi:10.1163/157075607780377965. https://www.researchgate.net/publication/233602810. Retrieved 2018-11-18.
- ↑ Leonard, J. L. (5 May 2006). "Sexual selection: lessons from hermaphrodite mating systems". Integrative and Comparative Biology 46 (4): 349–367. doi:10.1093/icb/icj041. PMID 21672747.
- ↑ For example, masturbation, trial mounting, and other behaviours are regularly seen in male animals out of season
- ↑ Denmark, Det Dyreetiske Råd, Udtalelse om menneskers seksuelle omgang med dyr (Copenhagen: Justitsministeriet, November 2006), p. 24. "Slimhinden i hundyrets vagina og dyrets adfærd er under indflydelse af dets brunstcyklus. Det betyder, at dyret er fysisk og mentalt mere parat til seksuelle aktiviteter på nogle tidspunkter end på andre. Men dette er ikke ensbetydende med, at den seksuelle aktivitet vil være forbundet med skader, angst og lidelse, hvis den foregår udenfor brunstperioden." (Translation: "The mucous membrane in the female animal's vagina and the animal's behaviour is under influence of its rut cycle. That means that the animal is physically and mentally more ready for sexual activities at some times than at others. But this does not mean that sexual activity will lead to injuries, fear or suffering, if it happens outside the rut period.")
- ↑ Virginia Douglass Hayssen; Ari Van Tienhoven (1993). Asdell's Patterns of Mammalian Reproduction: A Compendium of Species-specific Data. Cornell University Press. ISBN 978-0-8014-1753-5. https://books.google.com/books?id=yQzSe71g2AcC&q=spines. Retrieved 27 September 2013.
- ↑ Aronson, L. R.; Cooper, M. L. (1967). "Penile spines of the domestic cat: their endocrine-behavior relations.". Anatomical Record 157 (1): 71–78. doi:10.1002/ar.1091570111. PMID 6030760. http://www.catcollection.org/files/PenileSpines.pdf.
- ↑ Wells, Kentwood D (November 1978). "Territoriality in the green frog (Rana clamitans): Vocalizations and agonistic behaviour". Animal Behaviour 26 (4): 1051–1054. doi:10.1016/0003-3472(78)90094-5.
- ↑ Jump up to: 40.0 40.1 Georgiadis, J. R.; Kringelbach, M. L.; Pfaus, J. G. (2012). "Sex for fun: a synthesis of human and animal neurobiology". Nature Reviews Urology 9 (9): 486–498. doi:10.1038/nrurol.2012.151. PMID 22926422.
- ↑ Bear, Mark F. (2007). Neuroscience, exploring the brain. Baltimore, MD: Lippincott Williams and Wilkins. pp. 544–545. ISBN 978-0781760034. https://archive.org/details/neuroscienceexpl00mark.
- ↑ Inoue, K.; Burkett, J. P.; Young, L. J. (2013). "Neuroanatomical distribution of μ-opioid receptor mRNA and binding in monogamous prairie voles (Microtus ochrogaster) and non-monogamous meadow voles (Microtus pennsylvanicus)". Neuroscience 244: 122–133. doi:10.1016/j.neuroscience.2013.03.035. PMID 23537838.
- ↑ Jump up to: 43.0 43.1 Kennett, J.E.; McKee, D.T. (2012). "Oxytocin: An emerging regulator of prolactin secretion in the female rat". Journal of Neuroendocrinology 24 (3): 403–412. doi:10.1111/j.1365-2826.2011.02263.x. PMID 22129099.
- ↑ Snowdon, Charles T.; Pieper, Bridget A.; Boe, Carla Y.; Cronin, Katherine A.; Kurian, Aimee V.; Ziegler, Toni E. (2010). "Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins". Hormones and Behavior 58 (4): 614–618. doi:10.1016/j.yhbeh.2010.06.014. PMID 20600045.
- ↑ Lambert, K. (2011). The Lab Rat Chronicles. New York: penguin group. pp. 151–172.
- ↑ Azar, B. (March 2011). "Oxytocin's other side". Science Watch 42 (3): 40. http://www.apa.org/monitor/2011/03/oxytocin.aspx. Retrieved 4 November 2012.
- ↑ Aubert, Y.; Gustison, M. L.; Gardner, L. A.; Bohl, M. A.; Lange, J. R.; Allers, K. A.; Sommer, B.; Datson, N. A. et al. (2012). "Flibanserin and 8-OH-DPAT Implicate Serotonin in Association between Female Marmoset Monkey Sexual Behavior and Changes in Pair-Bond Quality". The Journal of Sexual Medicine 9 (3): 694–707. doi:10.1111/j.1743-6109.2011.02616.x. PMID 22304661.
- ↑ Gil, M.; Bhatt, R.; Picotte, K. B.; Hull, E. M. (2011). "Oxytocin in the medial preoptic area facilitates male sexual behavior in the rat". Hormones and Behavior 59 (4): 435–443. doi:10.1016/j.yhbeh.2010.12.012. PMID 21195714.
- ↑ Scott, Graham (2004). Essential Animal Behavior. Wiley-Blackwell. pp. 166–197. ISBN 978-0632057993.
- ↑ Agrati, D.; Fernández-Guasti, A.; Ferreño, M.; Ferreira, A. (2011). "Coexpression of sexual behavior and maternal aggression: The ambivalence of sexually active mother rats toward male intruders". Behavioral Neuroscience 125 (3): 446–451. doi:10.1037/a0023085. PMID 21517149.
- ↑ McHenry, J. A.; Bell, G. A.; Parrish, B. P.; Hull, E. M. (2012). "Dopamine D1 receptors and phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in the medial preoptic area are involved in experience-induced enhancement of male sexual behavior in rats". Behavioral Neuroscience 126 (4): 523–529. doi:10.1037/a0028707. PMID 22708956.
- ↑ Matsushita, Hiroaki; Tomizawa, Kazuhito; Okimoto, Naoki; Nishiki, Tei-ichi; Ohmori, Iori; Matsui, Hideki (2010-10-01). "Oxytocin mediates the antidepressant effects of mating behavior in male mice" (in en). Neuroscience Research 68 (2): 151–153. doi:10.1016/j.neures.2010.06.007. ISSN 0168-0102. PMID 20600375. https://www.sciencedirect.com/science/article/pii/S0168010210001641.
- ↑ Phan, Joseph; Alhassen, Lamees; Argelagos, Allan; Alhassen, Wedad; Vachirakorntong, Benjamin; Lin, Zitong; Sanathara, Nayna; Alachkar, Amal (2020-08-12). "Mating and parenting experiences sculpture mood-modulating effects of oxytocin-MCH signaling" (in en). Scientific Reports 10 (1): 13611. doi:10.1038/s41598-020-70667-x. ISSN 2045-2322. PMID 32788646. Bibcode: 2020NatSR..1013611P.
- ↑ Matsushita, Hiroaki; Sasaki, Yuya; Yunoki, Aya; Matsuji, Ayuka; Latt, Hein Min; Onishi, Kazunari; Tomizawa, Kazuhito; Matsui, Hideki (2022-08-01). "Antidepressant-like effect of male mating behavior through oxytocin-induced CREB signaling" (in en). Neuroscience Research 181: 74–78. doi:10.1016/j.neures.2022.04.002. ISSN 0168-0102. PMID 35421523. https://www.sciencedirect.com/science/article/pii/S0168010222001092.
- ↑ Hull, Elaine M.; Meisel, R. L.; Sachs, B. D. (2002). "Male sexual behavior". Hormones, Brain and Behavior 1: 3–137. doi:10.1016/B978-012532104-4/50003-2. ISBN 9780125321044. http://www.elaine-m-hull.com/publications/male_sex_beh_chap.pdf. Retrieved 13 August 2016.
- ↑ Jeffrey Moussaieff Masson (21 October 2009). When Elephants Weep: The Emotional Lives of Animals. Random House Publishing Group. ISBN 978-0-307-57420-6. https://books.google.com/books?id=TqxjMVBNEAsC&q=hyena%20ejaculated. Retrieved 28 May 2013.
- ↑ Balcombe, J. (2009). "Animal pleasure and its moral significance". Applied Animal Behaviour Science 118 (3): 212. doi:10.1016/j.applanim.2009.02.012. https://animalstudiesrepository.org/acwp_asie/5. Retrieved 6 July 2019.
- ↑ Dawkins, M. (2000). "Animal minds and animal emotions". American Zoologist 40 (6): 883–888. doi:10.1668/0003-1569(2000)040[0883:amaae2.0.co;2]. https://animalstudiesrepository.org/cgi/viewcontent.cgi?article=1004&context=emotio.[yes|permanent dead link|dead link}}]
- ↑ Panksepp, J. (1982). "Toward a general psychobiological theory of emotions". Behavioral and Brain Sciences 5 (3): 407–422. doi:10.1017/S0140525X00012759.
- ↑ "Emotions help animals to make choices (press release)". University of Bristol. 2010. http://www.bristol.ac.uk/news/2010/7165.html.
- ↑ Animals, Ethics and Trade: The Challenge of Animal Sentience. Earthscan. 2006. ISBN 9781844072545. https://books.google.com/books?id=Jl6cejHNQQUC&q=animal%20emotions&pg=PA28. Retrieved 26 October 2013.
- ↑ Denmark, Det Dyreetiske Råd, Udtalelse om menneskers seksuelle omgang med dyr (Copenhagen: Justitsministeriet, November 2006), p. 23–24. "Selv om det evolutionsmæssige formål med at parre sig kan siges at være reproduktion, er det ikke selve det, at dyrene får afkom, der i første omgang får dem til at parre sig. Det er til gengæld sandsynligt, at de parrer sig, fordi de er motiverede for selve parringsakten, og at denne er forbundet med en positiv oplevelse. Det er derfor rimeligt at antage, at der er en eller anden form for behag eller tilfredsstillelse forbundet med akten. Denne antagelse bekræftes af adfærden hos handyr, der for mange arters vedkommende er parate til at arbejde for at få adgang til hundyr, især hvis hundyret er i brunst, og handyr der i avlsøjemed er vant til at få tappet sæd – de viser stor ivrighed, når det udstyr, de forbinder med sædopsamlingen, tages frem. . . . Der er intet ved hunpattedyrenes anatomi eller fysiologi, der modsiger, at stimulation af kønsorganerne og parring skulle kunne være en positiv oplevelse – fx fungerer klitoris på samme måde som hos kvinder. Videnskabelige undersøgelser har vist, at reproduktionssuccesen forbedres ved stimulation af klitoris på bl.a. køer og hopper i forbindelse med insemination, fordi det forbedrer sædtransporten pga. sammentrækninger af de indre kønsdele. Dette gælder sandsynligvis også hundyr af andre dyrearter, og sammentrækninger i de indre kønsdele ses fx også under orgasme hos kvinder. Det er derfor rimeligt at antage, at det seksuelle samvær kan være forbundet med en positiv oplevelse for hundyrene."
- ↑ Jump up to: 63.0 63.1 Koeslag, J.H. (1990). "Koinophilia groups sexual creatures into species, promotes stasis, and stabilizes social behaviour". Journal of Theoretical Biology 144 (1): 15–35. doi:10.1016/S0022-5193(05)80297-8. PMID 2200930. Bibcode: 1990JThBi.144...15K.
- ↑ Moller, A.P.; Pomiankowski, A (1993). "Fluctuating asymmetry and sexual selection". Genetica 89 (1–3): 267–279. doi:10.1007/bf02424520.
- ↑ Dawkins, Richard (1986). The Blind Watchmaker. Longman, London. Published in Penguin Books 1988, 1991, and 2006. Chapter 8, Explosions and Spirals.
- ↑ Jump up to: 66.0 66.1 66.2 "1,500 animal species practice homosexuality". News-medical.net. 23 October 2006. http://www.news-medical.net/news/2006/10/23/1500-animal-species-practice-homosexuality.aspx.
- ↑ Katz, L. S.; McDonald, T. J. (1992). "Sexual behavior of farm animals". Theriogenology 38 (2): 239–253. doi:10.1016/0093-691X(92)90233-H. PMID 16727133.
- ↑ Knobil E., Neill J.D. (Eds). The physiology of reproduction. Academic Press, 3nd edition, 2005
- ↑ Streak spawning Fishbase Glossary. Retrieved 11 February 2011.
- ↑ Spawning rush Fishbase Glossary. Retrieved 11 February 2011.
- ↑ Gross MR (1984) "Sunfish, salmon, and the evolution of alternative reproductive strategies and tactics in fishes". Pages 55–75 in GW Potts and RJ Wottoon, eds. Fish reproduction: Strategies and tactics. Academic Press.
- ↑ Jump up to: 72.0 72.1 Shapiro DY (1984) "Sex reversal and sociodemographics processes in coral reef fishes" Pages 103–116 in GW Potts and RK Wootoon, eds., Fish reproduction: Strategies and tactics, Academic Press.
- ↑ Robertson, D.R.; R.R. Warner (1978). "Sexual patterns in the labroid fishes of the Western Caribbean II: the parrotfishes (Scaridae)". Smithsonian Contributions to Zoology 255 (255): 1–26. doi:10.5479/si.00810282.255.
- ↑ Kazancioglu, E.; S.H. Alonzo (August 2010). "A comparative analysis of sex change in Labridae supports the size advantage hypothesis". Evolution 64 (8): 2254–2264. doi:10.1111/j.1558-5646.2010.01016.x. PMID 20394662.
- ↑ Buston, Peter M. (May 2004). "Territory Inheritance in Clownfish". Proceedings of the Royal Society B 271 (Suppl 4): S252–S254. doi:10.1098/rsbl.2003.0156. PMID 15252999.
- ↑ Buston, P. (2004). "Does the Presence of Non-Breeders Enhance the Fitness of Breeders? An Experimental Analysis in the Clown Anemonefish Amphiprion percula". Behavioral Ecology and Sociobiology 57 (1): 23–31. doi:10.1007/s00265-004-0833-2.
- ↑ Colin, P.L.; L. J. Bell (1992). "Aspects of the spawning of labrid and scarid fishes (Pisces, Labroidei) at Enewetak Atoll, Marshall Islands with notes on other families (corrected reprint.)". Environmental Biology of Fishes 33 (3): 330–345. doi:10.1007/BF00005881.
- ↑ Zuk, Marlene (December 2016). "Mates with Benefits: When and How Sexual Cannibalism Is Adaptive". Current Biology 26 (23): R1230–R1232. doi:10.1016/j.cub.2016.10.017. PMID 27923131.
- ↑ Jump up to: 79.0 79.1 Polis, G.A. (1981). "The evolution and dynamics of intraspecific +4193 predation". Annual Review of Ecology and Systematics 51: 225–251. doi:10.1146/annurev.es.12.110181.001301.
- ↑ Bilde, T.; Tuni, C.; Elsayed, R.; Pekár, S.; Toft, S. (2006). "Death feigning in the face of sexual cannibalism". Biology Letters 2 (1): 23–5. doi:10.1098/rsbl.2005.0392. PMID 17148316.
- ↑ Bailey, R.O.; Seymour, N. R.; Stewart, G.R. (1978). "Rape behaviour in blue-winged teal". Auk 95 (1): 188–90. doi:10.2307/4085514. http://sora.unm.edu/sites/default/files/journals/auk/v095n01/p0188-p0190.pdf. Retrieved 5 March 2013.
- ↑ Barash, D. P. (1977). "Sociobiology of Rape in Mallards (Anas platyrhynchos): Responses of the Mated Male". Science 197 (4305): 788–789. doi:10.1126/science.197.4305.788. PMID 17790773. Bibcode: 1977Sci...197..788B.
- ↑ Mineau, Pierre; Cooke, Fred (1979). "Rape in the Lesser Snow Goose". Behaviour 70 (3): 280–291. doi:10.1163/156853979x00098. ISSN 0005-7959.
- ↑ Emlen, S. T.; Wrege, P. H. (2010). "Forced Copulations and Intra-specific Parasitism: Two Costs of Social Living in the White-fronted Bee-eater". Ethology 71: 2–29. doi:10.1111/j.1439-0310.1986.tb00566.x.
- ↑ Siebert, Charles. (8 October 2006) An Elephant Crackup?, Charles Siebert, New York Times Magazine, 8 October 2006 . Nytimes.com. Retrieved on 2011-12-22.
- ↑ "Have elephants begun raping rhinos in the wild?". 25 July 2008. http://www.straightdope.com/columns/read/2775/are-elephants-in-the-wild-showing-newly-aggressive-behavior-including-rape.
- ↑ Wininger, J. David (2004), "Parthenogenetic Stem Cells", Handbook of Stem Cells, Elsevier, pp. 635–637, doi:10.1016/b978-012436643-5/50072-9, ISBN 9780124366435
- ↑ Hiskey, D. (2011-05-31). "New Mexico whiptail lizards are all females". http://www.todayifoundout.com/index.php/2011/05/the-new-mexico-whiptail-lizard-is-made-up-entirely-of-females/.
- ↑ "Evolution of an all-female fish, Menidia clarkhubbsi (Atherinidae)". Evolution 37 (4): 772–784. 1983. doi:10.2307/2407918. PMID 28568119.
- ↑ Harmon, K. (2010). "No sex needed: All-female lizard species cross their chromosomes to make babies.". Scientific American. http://www.scientificamerican.com/article/asexual-lizards/. Retrieved 13 February 2015.
- ↑ "Captive shark had 'virgin birth'". BBC News. 2007-05-23. http://news.bbc.co.uk/2/hi/science/nature/6681793.stm.
- ↑ "'Virgin birth' for aquarium shark". Metro.co.uk. 2008-10-10. http://www.metro.co.uk/weird/article.html?Virgin_birth_for_aquarium_shark&in_article_id=351241&in_page_id=2.
- ↑ Halliday, Tim R.; Adler, Kraig, eds (1986). Reptiles & Amphibians. Torstar Books. p. 101. ISBN 978-0-920269-81-7.
- ↑ Walker, Brian (2010-11-11). "Scientists discover unknown lizard species at lunch buffet". CNN. http://www.cnn.com/2010/LIVING/11/10/lizard.lunch.discovery/.
- ↑ "The genetics of maintenance of an all-male lineage in the Squalius alburnoides complex". Journal of Fish Biology 60 (3): 649–662. 2002. doi:10.1111/j.1095-8649.2002.tb01691.x.
- ↑ Kinsey, Alfred (1953). Sexual Behavior in the Human Female. W.B. Saunders Company. p. 504. https://archive.org/details/sexualbehaviorin00inst.
- ↑ "Penguins are turning to prostitution". BBC. 26 February 1998. http://news.bbc.co.uk/2/hi/asia-pacific/60302.stm.
- ↑ Connor, Steve (8 April 2009). "Sex for meat – how chimps seduce their mates". The Independent (London). https://www.independent.co.uk/news/science/sex-for-meat-ndash-how-chimps-seduce-their-mates-1665356.html.
- ↑ Det Dyreetiske Råd (2006). Udtalelse om menneskers seksuelle omgang med dyr. Justitsministeriet. http://www.justitsministeriet.dk/fileadmin/downloads/Pressemeddelelser2006/d.pdf. "'Selv om det evolutionsmæssige formål med at parre sig kan siges at være reproduktion, er det ikke selve det, at dyrene får afkom, der i første omgang får dem til at parre sig. Det er til gengæld sandsynligt, at de parrer sig, fordi de er motiverede for selve parringsakten, og at denne er forbundet med en positiv oplevelse. Det er derfor rimeligt at antage, at der er en eller anden form for behag eller tilfredsstillelse forbundet med akten. Denne antagelse bekræftes af adfærden hos handyr, der for mange arters vedkommende er parate til at arbejde for at få adgang til hundyr, især hvis hundyret er i brunst, og handyr der i avlsøjemed er vant til at få tappet sæd – de viser stor ivrighed, når det udstyr, de forbinder med sædopsamlingen, tages frem.' [Translation to English] 'Although the evolutionary purpose of mating can be said to be reproduction, it is not the very fact that animals have offspring that causes them to mate in the first place. Rather, it is likely that they mate because they are motivated by the act of mating itself and that this is associated with a positive experience. It is therefore reasonable to assume that there is some form of pleasure or satisfaction associated with the act. This assumption is confirmed by the behaviour of male animals, which for many species are prepared to work to gain access to females, especially if the female is in heat, and male animals which for breeding purposes are used to having their semen collected - they show great eagerness when the equipment they associate with semen collection is taken out.'".
- ↑ Jump up to: 100.0 100.1 Pfaus, J. G.; Kippin, T. E.; Coria-Avila, G. A.; Gelez, H.; Afonso, V. M.; Ismail, N.; Parada, M. (2012). "Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance". Archives of Sexual Behavior 41 (1): 31–62. doi:10.1007/s10508-012-9935-5. PMID 22402996.
- ↑ Deaner M. O.; Khera A. V.; Platt M. L. (2005). "Monkeys pay per view: adaptive valuation of social images by rhesus macaques". Current Biology 15 (6): 543–548. doi:10.1016/j.cub.2005.01.044. PMID 15797023.
- ↑ Gray, Denis D. (27 November 2006) Porn sparks panda baby boom in China: Research — and blue movies — attributed to record-high birth rate in 2006 . Associated Press (via NBC News). Retrieved on 22 December 2011.
- ↑ Klaus Reinhardt, Nils Anthes, and Rolanda Lange (2015) "Copulatory Wounding and Traumatic Insemination" Cold Spring Harb Perspect Biol 2015; 7: a017582
- ↑ Watson, P. F. (1978). Artificial breeding of non-domestic animals: (the proceedings of a symposium held at the Zoological Society of London on 7 and 8 September 1977). Academic Press for the Zoological Society of London. ISBN 978-0-12-613343-1. https://books.google.com/books?id=kY8sZ7h4AEMC. Retrieved 9 February 2013.
- ↑ Balcombe, Jonathan P. (2011). The Exultant Ark: A Pictorial Tour of Animal Pleasure. University of California Press. pp. 89–. ISBN 978-0-520-26024-5. https://books.google.com/books?id=tz9mSyTWh0oC&pg=PA89.
- ↑ These Bears Are Having Lots Of Oral Sex, And Scientists Think They Know Why (The Huffington Post) By: Grenoble, Ryan.
- ↑ Hideshi Ogawa (2006). Wily Monkeys: Social Intelligence of Tibetan Macaques. Kyoto University Press. pp. 4–. ISBN 978-1-920901-97-4. https://books.google.com/books?id=EaKFLgm1TMUC&q=%22penis%22&pg=PA4.
- ↑ Fox, M. W. (1972). "The Social Significance of Genital Licking in the Wolf, Canis lupus". Journal of Mammalogy 53 (3): 637–640. doi:10.2307/1379064.
- ↑ Sugita, Norimasa. "Homosexual Fellatio: Erect Penis Licking between Male Bonin Flying Foxes Pteropus pselaphon." PLOS One 11.11 (2016): e0166024.
- ↑ Tan, M.; Jones, G.; Zhu, G.; Ye, J.; Hong, T.; Zhou, S.; Zhang, S.; Zhang, L. (2009). Hosken, David. ed. "Fellatio by Fruit Bats Prolongs Copulation Time". PLOS ONE 4 (10): e7595. doi:10.1371/journal.pone.0007595. PMID 19862320. Bibcode: 2009PLoSO...4.7595T.
- ↑ Waterman, J. M. (2010). Briffa, Mark. ed. "The Adaptive Function of Masturbation in a Promiscuous African Ground Squirrel". PLOS ONE 5 (9): e13060. doi:10.1371/journal.pone.0013060. PMID 20927404. Bibcode: 2010PLoSO...513060W.
- ↑ Tan, Min; Gareth Jones; Guangjian Zhu; Jianping Ye; Tiyu Hong; Shanyi Zhou; Shuyi Zhang; Libiao Zhang (28 October 2009). Hosken, David. ed. "Fellatio by Fruit Bats Prolongs Copulation Time". PLOS ONE 4 (10): e7595. doi:10.1371/journal.pone.0007595. PMID 19862320. Bibcode: 2009PLoSO...4.7595T.
- ↑ Bagemihl, Bruce (1999). Biological Exuberance: Animal Homosexuality and Natural Diversity. St. Martin's Press. p. 673. https://archive.org/details/biologicalexuber00bage.
- ↑ "Oslo gay animal show draws crowds". BBC News. 19 October 2006. http://news.bbc.co.uk/2/hi/europe/6066606.stm.
- ↑ Sazima, I. (2015). "Corpse bride irresistible: a dead female tegu lizard (Salvator merianae) courted by males for two days at an urban park in South-eastern Brazil". Herpetology Notes 8: 15–18. http://www.biotaxa.org/hn/article/download/9131/11610. Retrieved 24 February 2015.
- ↑ Jump up to: 116.0 116.1 116.2 de Waal FB (1995). "Bonobo sex and society". Scientific American 272 (3): 82–8. doi:10.1038/scientificamerican0395-82. PMID 7871411. Bibcode: 1995SciAm.272c..82W. "Perhaps the bonobo's most typical sexual pattern, undocumented in any other primate, is genito-genital rubbing (or GG rubbing) between adult females. One female facing another clings with arms and legs to a partner that, standing on both hands and feet, lifts her off the ground".
- ↑ Paoli, T.; Palagi, E.; Tacconi, G.; Tarli, S. B. (2006). "Perineal swelling, intermenstrual cycle, and female sexual behavior in bonobos (Pan paniscus)". American Journal of Primatology 68 (4): 333–347. doi:10.1002/ajp.20228. PMID 16534808.
- ↑ Walker, Matt (2 May 2008) Science/Nature 'Sex pest' seal attacks penguin . BBC News. Retrieved on 15 February 2011.
- ↑ Doncarlos, Michael W.; Petersen, Jay S.; Tilson, Ronald L. (1986). "Captive biology of an asocial mustelid; Mustela erminea". Zoo Biology 5 (4): 363–370. doi:10.1002/zoo.1430050407.
- ↑ Dawkins, Richard (2004). "Chimpanzees". The Ancestor's Tale. Houghton Mifflin. ISBN 978-1-155-16265-2.
- ↑ de Mattos Brito, L. B.; Joventino, I. R.; Ribeiro, S. C.; Cascon, P. (2012). "Necrophiliac behavior in the "cururu" toad, Rhinella jimi Steuvax, 2002, (Anura, Bufonidae) from Northeastern Brazil". North-Western Journal of Zoology 8 (2): 365. http://biozoojournals.ro/nwjz/content/v8n2/nwjz.121206.Brito.pdf. Retrieved 5 February 2015.
- ↑ Thornhill, R.; Gangestad, S. W. (2008). The Evolutionary Biology of Human Female Sexuality. USA: Oxford University Press. pp. 37–55. https://archive.org/details/evolutionarybiol00thor.
- ↑ Jump up to: 123.0 123.1 Simon De Bruxelles Promiscuous and bisexual — the 'faithful' seahorse has a secret sex life . Timesonline.co.uk. 31 January 2007. Retrieved on 22 December 2011.
- ↑ Zihlman, A. L.; Hunter, W. S. (1972). "A biomechanical interpretation of the pelvis of Australopithecus". Folia Primatologica 18 (1): 1–19. doi:10.1159/000155465. PMID 4658666.
- ↑ Jump up to: 125.0 125.1 Hashimoto, Chie (1997). "Context and Development of Sexual Behavior of Wild Bonobos (Pan paniscus) at Wamba, Zaire". International Journal of Primatology 18 (1): 1–21. doi:10.1023/a:1026384922066. ISSN 0164-0291.
- ↑ Clay, Zanna; de Waal, Frans B.M (2014). "Sex and strife: post-conflict sexual contacts in bonobos". Behaviour 152 (3–4): 313–334. doi:10.1163/1568539X-00003155. https://www.emory.edu/LIVING_LINKS/publications/articles/Clay_deWaal_2014.pdf. Retrieved 18 November 2018.
- ↑ Jump up to: 127.0 127.1 127.2 127.3 Leca, Jean-Baptiste; Gunst, Noëlle; Vasey, Paul L. (2014-05-28). "Male Homosexual Behavior in a Free-Ranging All-Male Group of Japanese Macaques at Minoo, Japan". Archives of Sexual Behavior 43 (5): 853–861. doi:10.1007/s10508-014-0310-6. ISSN 0004-0002. PMID 24867180.
- ↑ Jump up to: 128.0 128.1 Wallen, K.; Parsons, W. A. (1997). "Sexual behavior in same-sexed nonhuman primates: Is it relevant to understanding human homosexuality?". Annual Review of Sex Research 8: 195–223. PMID 10051894. https://www.researchgate.net/publication/13238242. Retrieved 2018-11-18.
- ↑ Vasey, Paul L.; Duckworth, Nadine (2006). "Sexual Reward via Vulvar, Perineal, and Anal Stimulation: A Proximate Mechanism for Female Homosexual Mounting in Japanese Macaques | Request PDF" (in en). Archives of Sexual Behavior 35 (5): 523–532. doi:10.1007/s10508-006-9111-x. PMID 17048107. https://www.researchgate.net/publication/6747847. Retrieved 2018-11-18.
- ↑ Jump up to: 130.0 130.1 Central Park Zoo's gay penguins ignite debate . Sfgate.com (7 February 2004). Retrieved on 22 December 2011.
- ↑ Jump up to: 131.0 131.1 Unwin, Brian (22 January 2008). "'Tougher laws' to protect friendly dolphins". The Telegraph (London). https://www.telegraph.co.uk/earth/earthnews/3322580/Tougher-laws-to-protect-friendly-dolphins.html.
- ↑ East, Marion L.; Hofer, Heribert; Wickler, Wolfgang (1993). "The erect 'penis' is a flag of submission in a female-dominated society: greetings in Serengeti spotted hyenas". Behavioral Ecology and Sociobiology 33 (6): 355–370. doi:10.1007/BF00170251.
- ↑ Sapolsky (1998), Why Zebras Don't Get Ulcers, W.H. Freeman and Co., ISBN:0-7167-3210-6, pp. 127–129.
- ↑ Dewsbury, Donald A. "Patterns of copulatory behavior in male mammals." Quarterly Review of Biology (1972): 1-33.
- ↑ Fortey, I. (2008). "The 15 most bizarre animal mating rituals". http://www.cracked.com/article_15926_the-15-most-bizarre-animal-mating-rituals.html.
- ↑ Dufour, Claire M.-S.; Pillay, Neville; Ganem, Guila (2015). "Ventro–ventral copulation in a rodent: a female initiative? | Journal of Mammalogy | Oxford Academic". Journal of Mammalogy 96 (5): 1017–1023. doi:10.1093/jmammal/gyv106.
- ↑ Diaz, K.. "Tursiops aduncus Indo-Pacific bottlenose dolphin". Animal Diversity Web. http://animaldiversity.org/site/accounts/information/Tursiops%20aduncus.html.
- ↑ Adulyanukosol, K.; Thongsukdee, S.; Hara, T.; Arai, N.; Tsuchiya, M. (2007). "Observations of dugong reproductive behavior in Trang Province, Thailand: further evidence of intraspecific variation in dugong behavior". Marine Biology 151 (5): 1887–1891. doi:10.1007/s00227-007-0619-y. Bibcode: 2007MarBi.151.1887A.
- ↑ "Bactrian & Dromedary Camels". Factsheets. San Diego Zoo Global Library. March 2009. http://library.sandiegozoo.org/factsheets/camel/camel.htm.
- ↑ Encyclopedia of Behavioral Neuroscience. Elsevier Science. 16 April 2010. ISBN 978-0-08-091455-8. https://books.google.com/books?id=DfV-BwAAQBAJ&q=multiple%20intromission.
- ↑ L. Alterman; Gerald A. Doyle; M.K. Izard (9 March 2013). Creatures of the Dark. Springer Science & Business Media. ISBN 978-1-4757-2405-9. https://books.google.com/books?id=0U_uBwAAQBAJ&q=copulation%20OR%20intromission%20OR%20thrusting.
- ↑ David E. Noakes (23 April 2009). Arthur's Veterinary Reproduction and Obstetrics. Elsevier Health Sciences UK. pp. 714–. ISBN 978-0-7020-3990-4. https://books.google.com/books?id=W5TdAwAAQBAJ&q=%22ejaculatory%20thrust%22.
- ↑ Donald M. Broom; Andrew Ferguson Fraser (1 January 2007). Domestic Animal Behaviour and Welfare. CABI. pp. 156–. ISBN 978-1-84593-287-9. https://books.google.com/books?id=2XcMielOfHAC&q=mating%20mounting%20thrusting&pg=PA156.
- ↑ Valerius Geist (January 1998). Deer of the World: Their Evolution, Behaviour, and Ecology. Stackpole Books. pp. 72–. ISBN 978-0-8117-0496-0. https://books.google.com/books?id=bcWZX-IMEVkC&q=(copulation%20OR%20coital%20OR%20intromission%20OR%20copulatory%20OR%20precopulatory%20OR%20postcopulatory%20OR%20mounting%20OR%20ejaculation)&pg=PA26.
- ↑ David M. Shackleton; Royal British Columbia Museum (1999). Hoofed Mammals of British Columbia. UBC Press. ISBN 978-0-7748-0728-9. https://books.google.com/books?id=iQq2iFktrwoC&q=(copulation%20OR%20coital%20OR%20intromission%20OR%20courtship%20OR%20copulatory%20OR%20precopulatory%20OR%20postcopulatory%20OR%20sexual%20OR%20mounting%20OR%20penis%20OR%20genitalia%20OR%20ejaculation).
- ↑ Lührs, Mia-Lana; Kappeler, Peter M. (2014). "Polyandrous mating in treetops: How male competition and female choice interact to determine an unusual carnivore mating system". Behavioral Ecology and Sociobiology 68 (6): 879–889. doi:10.1007/s00265-014-1701-3.
- ↑ Dixson, A. F. (1995). "Baculum length and copulatory behaviour in carnivores and pinnipeds (Grand Order Ferae)". Journal of Zoology 235 (1): 67–76. doi:10.1111/j.1469-7998.1995.tb05128.x. https://www.researchgate.net/publication/229990265. Retrieved 27 August 2020.
- ↑ Tatara, Masaya (1994). "Notes on the breeding ecology and behavior of Japanese martens on Tsushima Islands, Japan". Journal of the Mammalogical Society of Japan 19 (1): 67–74. https://scholar.google.com/scholar?start=10&q=%22copulatory+(tie%7Clock)%22. Retrieved 27 June 2017.
- ↑ Dixson, Alan F (1987). "Baculum length and copulatory behavior in primates". American Journal of Primatology 13 (1): 51–60. doi:10.1002/ajp.1350130107. PMID 31973483. https://www.researchgate.net/publication/247964388.
- ↑ Møller, A. P.; Birkhead, T. R. (1989). "Copulation behaviour in mammals: evidence that sperm competition is widespread". Biological Journal of the Linnean Society 38 (2): 119–131. doi:10.1111/j.1095-8312.1989.tb01569.x. https://academic.oup.com/biolinnean/article-pdf/38/2/119/14071594/j.1095-8312.1989.tb01569.x.pdf.
- ↑ Rogers, David; Chase, Ronald (2001). "Dart receipt promotes sperm storage in the garden snail Helix aspersa". Behavioral Ecology and Sociobiology 50 (2): 122–7. doi:10.1007/s002650100345.
- ↑ Chase, R. (2007). "The function of dart shooting in helicid snails". American Malacological Bulletin 23: 183–189. doi:10.4003/0740-2783-23.1.183.
- ↑ Chase, R.; Blanchard, K. C. (2006). "The snail's love-dart delivers mucus to increase paternity". Proceedings of the Royal Society of London B: Biological Sciences 273 (1593): 1471–1475. doi:10.1098/rspb.2006.3474. PMID 16777740. PMC 1560308. https://www.mcgill.ca/newsroom/news/item/?item_id=19226. Retrieved 24 August 2017.
- ↑ Newman, Leslie. "Fighting to mate: flatworm penis fencing". PBS. https://www.pbs.org/kcet/shapeoflife/episodes/hunt_explo2.html.
- ↑ Jump up to: 155.0 155.1 Veron, J.E.N. (2000). Corals of the World. Vol 3 (3rd ed.). Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd.. ISBN 978-0-642-32236-4.
- ↑ Barnes, R. and; Hughes, R. (1999). An Introduction to Marine Ecology (3rd ed.). Malden, MA: Blackwell Science, Inc.. pp. 117–141. ISBN 978-0-86542-834-8.
- ↑ Hatta, M.; Fukami, H.; Wang, W.; Omori, M.; Shimoike, K.; Hayashibara, T.; Ina, Y.; Sugiyama, T. (1999). "Reproductive and genetic evidence for a reticulate evolutionary theory of mass spawning corals". Molecular Biology and Evolution 16 (11): 1607–1613. doi:10.1093/oxfordjournals.molbev.a026073. PMID 10555292.
- ↑ "Chan Lee Peng, August 2008". Scienceray.com. http://scienceray.com/biology/zoology/13-strangest-and-most-unusual-sexual-behavior-among-the-animals/2/.
- ↑ Jump up to: 159.0 159.1 159.2 "Sexual Appetite and Animal behaviour – Sex and the single spider". The Economist 400 (8742). 14 July 2011. http://www.economist.com/node/18956086.
- ↑ Bel-Venner, M.c; Dray, S; Allainé, D; Menu, F; Venner, S (2008-01-07). "Unexpected male choosiness for mates in a spider". Proceedings of the Royal Society B: Biological Sciences 275 (1630): 77–82. doi:10.1098/rspb.2007.1278. PMID 17956845.
- ↑ Bel-Venner, M. C.; Venner, S. (2006-06-01). "Mate-guarding strategies and male competitive ability in an orb-weaving spider: results from a field study" (in en). Animal Behaviour 71 (6): 1315–1322. doi:10.1016/j.anbehav.2005.08.010. ISSN 0003-3472. http://www.sciencedirect.com/science/article/pii/S0003347206000972.
- ↑ Patterson, Nick; Daniel J. Richter; Sante Gnerre; Eric S. Lander; David Reich (29 June 2006). "Genetic evidence for complex speciation of humans and chimpanzees". Nature 441 (7097): 1103–1108. doi:10.1038/nature04789. ISSN 0028-0836. PMID 16710306. Bibcode: 2006Natur.441.1103P.
- ↑ Wade, Nicholas (18 May 2006) Two Splits Between Human and Chimp Lines Suggested , New York Times.
- ↑ Yamamichi, M; Gojobori J; Innan H. (Jan 2012). "An autosomal analysis gives no genetic evidence for complex speciation of humans and chimpanzees". Mol Biol Evol 29 (1): 145–56. doi:10.1093/molbev/msr172. PMID 21903679.
- ↑ "The genetics of inbreeding depression". Nature Reviews Genetics 10 (11): 783–96. 2009. doi:10.1038/nrg2664. PMID 19834483.
- ↑ "Kin discrimination and female mate choice in the naked mole-rat Heterocephalus glaber". Proc. Biol. Sci. 266 (1432): 1995–2002. 1999. doi:10.1098/rspb.1999.0877. PMID 10584337.
- ↑ "An experimental study of inbreeding depression in a natural habitat". Science 266 (5183): 271–3. 1994. doi:10.1126/science.7939661. PMID 7939661. Bibcode: 1994Sci...266..271J.
- ↑ "The genetic basis of inbreeding avoidance in house mice". Curr. Biol. 17 (23): 2061–6. 2007. doi:10.1016/j.cub.2007.10.041. PMID 17997307.
- ↑ "Odour-based kin discrimination in the cooperatively breeding meerkat". Biology Letters 9 (1): 20121054. 2013. doi:10.1098/rsbl.2012.1054. PMID 23234867.
- ↑ "Inbreeding and inbreeding depression of early life traits in a cooperative mammal". Mol. Ecol. 21 (11): 2788–804. 2012. doi:10.1111/j.1365-294X.2012.05565.x. PMID 22497583. Bibcode: 2012MolEc..21.2788N.
- ↑ "Role of male-biased dispersal in inbreeding avoidance in the grey-sided vole (Myodes rufocanus)". Mol. Ecol. 17 (22): 4887–96. 2008. doi:10.1111/j.1365-294X.2008.03969.x. PMID 19140979.
- ↑ "Dispersal as a means of inbreeding avoidance in a wild bird population". Proc. Biol. Sci. 275 (1635): 703–11. 2008. doi:10.1098/rspb.2007.0989. PMID 18211876.
- ↑ Waldman, B; Rice, JE; Honeycutt, RL (1992). "Kin recognition and incest avoidance in toads". Am. Zool. 32: 18–30. doi:10.1093/icb/32.1.18.
Bibliography
- Bagemihl, Bruce (1999). Biological Exuberance: Animal Homosexuality and Natural Diversity. St. Martin's Press. ISBN 978-0-312-19239-6. https://books.google.com/books?id=tmFJ1LhbVWcC.
- Schaller, G.B. (1972). The Serengeti Lion. University of Chicago Press. ISBN 978-0226736600.
Further reading
- R. F. Ewer (11 December 2013). Ethology of Mammals. Springer. ISBN 978-1-4899-4656-0. https://books.google.com/books?id=0LTzBwAAQBAJ&q=mating%20OR%20intromission%20OR%20copulation%20OR%20coitus%20OR%20%22sexual%20behavior%22.
- Nadler, Ronald D. (1980). "Reproductive physiology and behaviour of gorillas". The Great Apes of Africa. Suppl 28. Cambridge: Journals of Reproduction and Fertility Ltd.. 59–70. ISBN 978-0906545041. https://pubmed.ncbi.nlm.nih.gov/6934312/.
- Richard Estes (1991). The Behavior Guide to African Mammals: Including Hoofed Mammals, Carnivores, Primates. University of California Press. ISBN 978-0-520-08085-0. https://books.google.com/books?id=g977LsZHpcsC&q=intromission%20OR%20copulation%20OR%20coitus%20OR%20ejaculation%20OR%20%22sexual%20behavior%22.
- William F. Perrin; Bernd Wursig; J.G.M. 'Hans' Thewissen (26 February 2009). Encyclopedia of Marine Mammals. Academic Press. ISBN 978-0-08-091993-5. https://books.google.com/books?id=2rkHQpToi9sC&q=mating%20OR%20intromission%20OR%20copulation%20OR%20coitus%20OR%20%22sexual%20behavior%22.
- John Vandenbergh (28 August 1983). Pheromones and Reproduction in Mammals. Elsevier. ISBN 978-0-323-15651-6. https://books.google.com/books?id=PUpYID8Rc3UC.
- Temple Grandin; Mark J. Deesing (22 April 2013). Genetics and the Behavior of Domestic Animals. Academic Press. ISBN 978-0-12-405508-7. https://books.google.com/books?id=4L3dMJ8-aWgC&q=ejaculation%20OR%20mating%20OR%20copulation%20OR%20intromission%20OR%20%22sexual%20behavior%22%20OR%20coitus.
- Menna Jones; Chris R. Dickman; Michael Archer (2003). Predators with Pouches: The Biology of Carnivorous Marsupials. Csiro Publishing. ISBN 978-0-643-06634-2. https://books.google.com/books?id=3YQSDiWHfD0C&q=mating%20OR%20intromission%20OR%20copulation%20OR%20coitus%20OR%20ejaculation%20OR%20%22sexual%20behavior%22%20OR%20estrus.
- Ernst Knobil (2006). Knobil and Neill's Physiology of Reproduction. Gulf Professional Publishing. ISBN 978-0-12-515402-4. https://books.google.com/books?id=11f2zMjqqVkC&q=(copulation%20OR%20coital%20OR%20intromission%20OR%20courtship%20OR%20copulatory%20OR%20precopulatory%20OR%20postcopulatory%20OR%20%22sexual%20behavior%22%20OR%20%22reproductive%20behavior%22%20OR%20mounting).
- Sexual behavior of horses
- Morel, M.C.G.D. (2008). Equine Reproductive Physiology, Breeding and Stud Management. CABI. ISBN 978-1-78064-073-0. https://books.google.com/books?id=iz1C6pSwq40C&q=(copulation%20OR%20coital%20OR%20intromission%20OR%20courtship%20OR%20copulatory%20OR%20precopulatory%20OR%20postcopulatory%20OR%20sexual%20OR%20mounting%20OR%20ejaculation).
- D. S. Mills; S. M. McDonnell (10 March 2005). The Domestic Horse: The Origins, Development and Management of Its Behaviour. Cambridge University Press. pp. 110–. ISBN 978-0-521-89113-4. https://books.google.com/books?id=GHKuEeqC4U0C&q=(copulation%20OR%20coital%20OR%20intromission%20OR%20courtship%20OR%20copulatory%20OR%20precopulatory%20OR%20postcopulatory%20OR%20sexual%20OR%20mounting%20OR%20penis%20OR%20genitalia%20OR%20ejaculation)&pg=PA110.
- Jonathan Pycock; Juan C. Samper; Angus O. McKinnon (23 November 2006). Current Therapy in Equine Reproduction E-Book. Elsevier Health Sciences. ISBN 978-1-4377-1300-8. https://books.google.com/books?id=d2ac2NPLxsQC&q=(copulation%20OR%20coital%20OR%20intromission%20OR%20courtship%20OR%20copulatory%20OR%20precopulatory%20OR%20postcopulatory%20OR%20sexual%20OR%20%22reproductive%20behavior%22%20OR%20mounting).
External links
- National Geographic
- San Francisco Zoo has run a "sex tour" covering animal sexuality, on Valentine's Day
- A wild, and gay, kingdom World Science
- Is it relevant to look at the animal kingdom to determine if human same-sex behaviour is "natural"?
 |








