Biology:Chaetomium elatum
| Chaetomium elatum | |
|---|---|
| |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Sordariomycetes |
| Order: | Sordariales |
| Family: | Chaetomiaceae |
| Genus: | Chaetomium |
| Species: | C. elatum
|
| Binomial name | |
| Chaetomium elatum Kunze (1818)
| |
| Synonyms | |
| |
Chaetomium elatum is a very common and widely distributed saprotrophic fungus of the Chaetomiaceae family of molds which has been found to grow on many different substances all over the world.[3][4][5][6] It was first established by Gustav Kunze after he observed it growing on dead leaves.[3][4] Its defining features that distinguish it from other Chaetomium species are its extremely coarse terminal hairs[7] and the lemon-shaped morphology of its ascospores.[4] It produces many metabolites with potential biotechnology uses including one with promise against the rice blast disease fungus, Magnaporthe grisea.[8] It shows very little pathogenic ability causing confirmed disease in only a few plant species.[9][10]
History and taxonomy
Gustav Kunze established the genus Chaetomium in 1817 after discovering a new species of fungus in dead stalks and leaves which he named C. globosum.[3][4] In 1818, when observing the dead leaves of Typha and Sparganium in Germany , Kunze recognized a new fungus that looked like C. globosum but was darker in pigmentation, and after characterizing it named it Ch. elatum.[3][4] In addition to Kunze's identification and characterization of the species (in which he failed to discern asci), Robert Greville created illustrations in 1826 to show the morphology of the species.[3][4] Despite this, C. elatum has been confused by other mycologists many times and thus has been re-described more than any other Chaetomium species, leading to many obligate synonyms.[3][4] It was during the creation of one of these synonyms, C. lageniforme, by August Corda in 1837 that asci were first recognized, thus identifying the defining feature that placed this fungus in the fungal division, Ascomycota.[4]
Growth and morphology
Chaetomium elatum produces darkly-coloured oval perithecia covered with stiff, black hairs.[4] The perithecia are typically attached firmly to the substratum by dark/black rhizoids.[3][4] In laboratory colonies C. elatum generally grows 5–6 mm per day,[11] but can show different growth rates and colour characteristics depending on the growth medium.[12] Under certain growth conditions, colonies of some strains of C. elatum may develop coloured guttation droplets of liquid on their surfaces whose function and composition are unknown.[12][13] C. elatum has a homothallic mating system.[6][14]
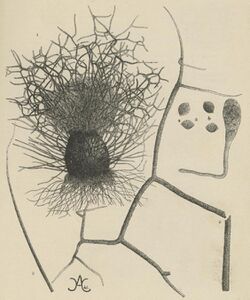
The perithecia are superficial, usually mature in 13 to 20 days, and are 280–440 μm high with a diameter of 255–380 μm.[11][13] They may appear greenish in color under reflected light with a round/oval-like shape and have an ostiole that is sparsely covered in white/buff aerial hyphae.[11][13] The perithecial wall is made of brown interwoven hyphae or tightly packed pseudoparenchyma.[11][13] Morphology of the black/dark perithecium hairs varies depending on their location.[3][4] Terminal hairs are extremely coarse, branched at right to straight angles, have irregular projections, blunt spines, and dwindle off to thin translucent tips.[3][4][13] Lateral hairs are thin, long, unbranched, coarsely roughened by irregular projections and dwindle into translucent smooth tips that are vaguely separate.[3][4] The difference between the terminal hair of C. elatum and C. globosum is a distinguishing factor between the two taxa.[7]
The asci of C. elatum are generally club-shaped and contain 8 round ascospores.[3][4] The ascospores are translucent/light olive when young and become brown with pointed tips when they mature giving them lemon-like shape when viewed in profile.[3][4][13] The ascospores also have a thick wall[11] with a small pore on the outer wall of their apex.[13][11] Morphology of the ascospores is a distinguishing factor when compared to other Chaetomium species with which it might be confused like C. indicum, C. funicolum, and C. virgecephalum.[4]
The asexual morph of C. elatum has acremonium-like growth, with its conidia being borne on phialidic conidiophogeous cells that form on aerial aseptate hyphae and are 6–24.5 μm long with a diameter of 1.5–3.5 μm at the base.[13] Conidium dimensions are 2.5–5.5 μm × 1.5–2.5 μm and they form towards the base of the conidiophore in chains, are translucent, smooth, and oval-shaped with a rounded apex and short base.[13] rhizo
Habitat and ecology
Chaetomium elatum is a very common and widely distributed species of Chaetomium, with it being found all over the world.[3][4] The species has been found in many areas of the United States , Canada , England , France , Russia , Switzerland , Germany , Scotland, the Galapagos Islands and many other localities.[3][4]
It is the most common species of fungi that grows on damp rotting straw,[6] but has also been found and isolated from a variety of materials like rope, burlap, wood, paper, cellulose products, animal dung, seeds, barrel hoops, old brooms, Hordeum vulgare L, Triticum aestivum and the dead leaves of Typha and Sparganium.[3][4][5] In general this species of Chaetomium mainly colonizes cereal, Alkali seepweed, True grasses,[9] has been found to interact with Japanese yew, Alkali seepweed, European rabbit, Bread wheat, True grasses, Corn.[9] It has also been associated with the mycobiota of Sugarcane[15] as well as is known as a root-colonizing fungus in the avocado plant where it serves as both a rhizoplane and rhizosphere.[10]
Biotechnology uses
Chaetomium elatum has been isolated from different materials[5] and its metabolic properties with potential biotechnology uses have been explored. In the presence of nitrocellulose (a very important cellulose derivative).[16]C. elatum can break down nitrocellulose in liquid culture.[16] Investigations into the types of metabolites produced by this fungus have found that it produces benzoquinone derivatives,[17] tetra-S-methyl derivatives,[17] anthraquinone-chromanone,[17] orsellinic acid,[17] globosumones,[17] sterols[17] Chaetoglobsins,[7][17] Cochliodones 1–3 (azaphilone derivatives[18]),[7] azaphilones,[19] chlorinated phenolic glycosides,[19] and xanthoquinodins.[20] Xanthoquinodins are fungal metabolites that have been found to have antibacterial, antifungal, anticoccidial, antiplasmodial, and cytotoxic activities.[20] Azaphilones have antimicrobial, antifungal, antiviral, antioxidant, cytotoxic, nematicidal and anti-inflammatory properties,[21] and the three metabolized by C. elatum have also been found to inhibit Caspase 3 which is involved in cell death.[19] Phenolic compounds have shown to possess antimicrobial properties.[22] Chaetoglobosins has been found to have anticancer activity,[23][17] and benzoquinone derivatives have antibacterial properties.[24] Nnanoparticles harvested from crude extracts of the C. elatum exhibit antimicrobial activity against Magnaporthe grisea, the plant pathogen that causes rice blast disease.[8]
Plant pathogenicity
Chaetomium elatum is a known pathogen of the common grape vine.[9] In 2007, an investigation to determine its pathogenicity on avocado plants found that it opportunistically colonizes the plant roots and only becomes pathogenic when resources are very limited and intraspecific competition is high.[10]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Species Fungorum". http://www.speciesfungorum.org.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Mycobank:Chaetomium elatum". http://www.mycobank.org/Biolomics.aspx?Table=Mycobank&MycoBankNr_=172050.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 Chivers, A.H. (10 June 1915). "A monograph of the genera Chaetomium and Ascotricha". Memoirs of the Torrey Botanical Club 14 (3): 155–240. doi:10.2307/3757086.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 Ames, LM (1961) (in English). A monograph of the chaetomiaceae. United States Army research and development series. 2. Durham, N.C.: Army Research Office. pp. 66. ISBN 9780000072641. https://babel.hathitrust.org/cgi/pt?id=coo.31924000643282;view=1up;seq=1.
- ↑ 5.0 5.1 5.2 "Global Catalogue of Microorganisms:Chaetomium elatum". http://gcm.wfcc.info/speciesPage.jsp?strain_name=Chaetomium%20elatum.
- ↑ 6.0 6.1 6.2 Caretta, G; Piontelli, E (1998). "Preserved ascomatal and other fungal structures on the remains of a ninth century Longobard abbess exhumed from a Monastery in Pavia, Italy". Mycopathologia 140 (2): 77–83. doi:10.1023/A:1006805226954. PMID 9646511.
- ↑ 7.0 7.1 7.2 7.3 Dosen, I; Nielsen, KF; Clausen, G; Andersen, B (2017). "Potentially harmful secondary metabolites produced by indoor Chaetomium species on artificially and naturally contaminated building materials.". Indoor Air 27 (1): 34–46. doi:10.1111/ina.12290. PMID 26880675. http://orbit.dtu.dk/ws/files/123080054/Potentially_harmful_secondary_metabolites_produced_by_indoor_Chaetomium_species_on_artificially_and_naturally_contaminated_building_materials.post_print.pdf.
- ↑ 8.0 8.1 Song, Jiaojiao; Soytong, Kasem; Kanokmedhakul, Somdej (2016). "Antifungal Activity of Chaetomium elatum against Pyricularia oryzae Causing Rice Blast". International Journal of Agricultural Technology 12 (7.1): 1437–1447. ISSN 1686-9141. http://www.ijat-aatsea.com/pdf/v12_n7_1_16_DecemberSpecialissue/Aricultural%2012no7.1%20(1439-1450)%202.pdf.
- ↑ 9.0 9.1 9.2 9.3 "Global Biotic Interactions:Chaetomium elatum". https://www.globalbioticinteractions.org/?interactionType=interactsWith&sourceTaxon=NCBI%3A5150.
- ↑ 10.0 10.1 10.2 Violi, HA; Menge, JA; Beaver, RJ (2007). "Chaetomium elatum (Kunze: Chaetomiaceae) as a root-colonizing fungus in avocado: is it a mutualist, cheater, commensalistic associate, or pathogen?". American Journal of Botany 94 (4): 690–700. doi:10.3732/ajb.94.4.690. PMID 21636437.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Encyclopedia of life:Chaetomium elatum". http://www.eol.org/pages/1029519/details.
- ↑ 12.0 12.1 Udagawa, SI (1960). "A Taxonomic Study on the Japanese Species of Chaetomium". J. Gen. Appl. Microbiol. 6 (4): 223–251. doi:10.2323/jgam.6.223. https://www.jstage.jst.go.jp/article/jgam1955/6/4/6_4_223/_pdf/-char/ja.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 Wang, X.W.; Houbraken, J; Groenewald, J.Z.; Meijer, M; Andersen, B; Nielsen, K.F.; Crous, P.W.; Samson, R.A. (2016). "Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments". Studies in Mycology 84 (1): 145–224. doi:10.1016/j.simyco.2016.11.005. PMID 28082757.
- ↑ Seth, H.K. (Aug 1967). Studies on the Genus Chaetomium. I. Heterothallism. 59. Taylor & Francis, Ltd.. 580–584. doi:10.1080/00275514.1967.12018450. ISBN 9781378318140.
- ↑ Abdullah, S.K.; Saleh, Y.A. (2010). "Mycobiota Associated with Sugarcane (Saccharum officinarum L.) Cultivars in Iraq.". Jordan Journal of Biological Sciences 3 (4): 193–202. ISSN 1995-6673.
- ↑ 16.0 16.1 Auer, N; Hedger, JN; Evans, CS (2005). "Degradation of nitrocellulose by fungi.". Biodegradation 16 (3): 229–236. doi:10.1007/s10532-004-0896-9. PMID 15865147.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Thohinung, S; Kanokmedhakul, s; Kanokmedhakul, K; Kukongviriyapan, V; Tusskorn, O; Soytong, K (2010). "Cytotoxic 10-(Indol-3-yl)-[13]cytochalasans from the Fungus Chaetomium elatum ChE01". Arch. Pharm. Res. 33 (8): 1135–1141. doi:10.1007/s12272-010-0801-5. PMID 20803114.
- ↑ Yu, FX; Chen, Y; Yang, YH; Zhao, PJ (2016). "Four new dimeric spiro-azaplilone derivatives cochliodones E-H from the entophytic fungus Chaetomium sp. M336". Phytochemistry Letters 16: 263–267. doi:10.1016/j.phytol.2016.05.003. Bibcode: 2016PChL...16..263Y.
- ↑ 19.0 19.1 19.2 Chen, GD; Li, YJ; Gao, H; Chen, Y; Li, XX; Li, J; Guo, LD; Cen, YZ et al. (2012). "New azaphilones and chlorinated phenolic glycosides from Chaetomium elatum with caspase-3 inhibitory activity.". Planta Medica 78 (15): 1683–1689. doi:10.1055/s-0032-1315211. PMID 22890540.
- ↑ 20.0 20.1 Chen, GD; Chen, Y; Gao, H; Shen, LQ; Wu, Y; Li, XX; Li, Y; Guo, LD et al. (2013). "Xanthoquinodins from the Endolichenic Fungal Strain Chaetomium elatum". J. Nat. Prod. 76 (4): 702–709. doi:10.1021/np400041y. PMID 23586920.
- ↑ Osmanova, N; Schultze, W; Ayoub, Nahla (2010). "Azaphilones: a class of fungal metabolites with diverse biological activities". Phytochemistry Reviews 9 (2): 315–342. doi:10.1007/s11101-010-9171-3. Bibcode: 2010PChRv...9..315O.
- ↑ Maddox, CE; Laur, LM; Tiam, Li (1973). "Antibacterial Activity of Phenolic Compounds Against the Phytopathogen Xylella fastidiosa". Current Microbiology 60 (1): 53–58. doi:10.1016/S0040-4039(01)86820-9. PMID 19813054.
- ↑ Sekita, S; Yoshihara, K; Kuwano, H (2010). "Structures of chaetoglobosin A and B, cytotoxic metabolites of Chaetomium globosum". Tetrahedron Lett 14 (23): 2109–2112. doi:10.1007/s00284-009-9501-0. PMID 19813054.
- ↑ Lana, EJL; Carazza, F; Takahashi, JA (2006). "Antibacterial Evaluation of 1,4-Benzoquinone Derivatives". J. Agric. Food Chem. 54 (6): 2053–2056. doi:10.1021/jf052407z. PMID 16536574.
Wikidata ☰ Q10447635 entry
 |

