Biology:Maltose-binding protein
| Maltose/maltodextrin-binding periplasmic protein | |||||||
|---|---|---|---|---|---|---|---|
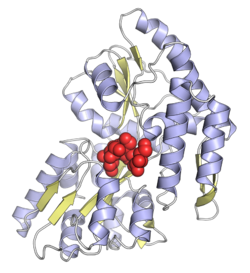 Maltose-binding protein from Escherichia coli, with a bound sugar molecule shown as red spheres, from PDB: 1FQC.[1] | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | MalE | ||||||
| UniProt | P0AEX9 | ||||||
| |||||||
Maltose-binding protein (MBP) is a part of the maltose/maltodextrin system of Escherichia coli, which is responsible for the uptake and efficient catabolism of maltodextrins. It is a complex regulatory and transport system involving many proteins and protein complexes. MBP has an approximate molecular mass of 42.5 kilodaltons.
Structure and folding
MBP is encoded by the malE gene of Escherichia coli. The malE gene codes for a precursor polypeptide (396 amino acid residues) which yields the mature MBP (370 residues) upon cleavage of the NH2-terminal extension (26 residues). The precursor and mature forms of MBP do not contain any cysteine residues.[2]
MBP is a monomeric protein. Crystal structures have shown that MBP is divided into two distinct globular domains that are connected by three short polypeptide segments. The two domains are separated by a deep groove that contains the maltose/maltodextrin binding site. Comparison of the structures of the liganded and unliganded forms of MBP has shown that the binding of maltose induces a major conformational change that closes the groove by a rigid motion of the two domains around the linking polypeptide hinge.[3][4]
Both precursor and mature forms of MBP are functional for the binding of maltose.[5] The NH2-terminal extension decreases the folding rate of the precursor form of MBP relative to its mature form by at least 5 fold, but it has no effect on the unfolding rate.[6][7] The equilibrium unfolding of MBP can be modelled by a two-state mechanism with a stability ∆G(H2O) equal to 9.45 kcal mol−1 at 25 °C, pH 7.6.[8]
Localization and export
MBP is exported into the periplasmic space of E. coli.[9] The NH2-terminal extension of MBP, also termed signal peptide, has two roles: (i) it slows down folding of the newly synthesized polypeptide, and (ii) it directs this polypeptide to the membrane and SecYEG translocon. Once folded, the precursor can no longer enter the translocation pathway.[10] The introduction of a charged amino-acid residue or a proline residue within the hydrophobic core of the signal peptide is sufficient to block export.[11] The defective exports of the mutant MBPs are consistent with the alpha-helical conformation and hydrophobic interactions of the signal peptide in its interaction with the translocon motor protein SecA.[12][13][14]
Control of expression
The malE gene, coding for MBP, belongs to the Mal regulon of E. coli, which consists of ten genes whose products are geared for the efficient uptake and utilization of maltose and maltodextrins. All the gene involved in the transport of maltose/maltodextrin, including malE, are clustered in the malB region of E. coli and organized in two divergent operons: malE-malF-malG and malK-lamB.[15] The transcription start sites at the malEp and malKp promoters are distant of 271 base pairs.[16]
The malEp and malKp promoters are synergistically activated by protein MalT, the activator of the Mal regulon and by the cAMP receptor protein CRP. This activation is a coupled process that involves, going from malEp towards malKp: two MalT binding sites; three CRP binding site, and two overlapping sets of three MalT binding sites, staggered by three base pairs.[16][17][18] Transcription activation requires the binding of adenosine triphosphate (ATP) and maltotriose to MalT and the binding of cyclic AMP to the dimer of CRP.[19] The unliganded form of MalT is monomeric whereas its liganded form, in the presence of ATP and maltotriose, is oligomeric.[20]
Use as a protein and peptide vector
MBP is used to increase the solubility of recombinant proteins expressed in E. coli. In these systems, the protein of interest is often expressed as a MBP-fusion protein, preventing aggregation of the protein of interest. The mechanism by which MBP increases solubility is not well understood. In addition, MBP can itself be used as an affinity tag for purification of recombinant proteins. The fusion protein binds to amylose columns while all other proteins flow through. The MBP-protein fusion can be purified by eluting the column with maltose. Once the fusion protein is obtained in purified form, the protein of interest is often cleaved from MBP with a specific protease and can then be separated from MBP by affinity chromatography.
A first study of the relations between structure and functions of MBP was performed by random insertion of a short DNA fragment, coding for a BamHI restriction site, into the malE gene. Some of the insertions affected the functions of MBP whereas others were permissive.[21][22] The permissive sites that were internal to MBP, were used to insert antigenic peptides and challenge the immune response in mice.[23] The 3'-OH terminal insertions were used to create fusion proteins and develop the use of MBP as an affinity handle for the purification of foreign proteins and peptides by affinity chromatography on cross-linked amylose and elution with maltose in mild physico-chemical conditions.[24][25] Several plasmid vectors were developed to facilitate the expression and purification of such fusion proteins.[26]
When the recombinant MBP includes a signal peptide, the fusion protein can be exported into the periplasmic space, which facilitate its purification since the periplasmic fluid contains only a limited number of proteins and can be recovered either by an osmotic shock or by permeabilization of the bacterial outer membrane with antibiotics such as Polymyxin B. Such an export of the fusion protein into the periplasmic space enables the formation of disulfide bonds in the passenger protein, for example antibody fragments.[27][28] Foreign proteins that are exported or secreted in their native organism, can usually be exported into the E. coli periplasm by fusion with MBP. Examples of cytoplasmic proteins that could be exported by fusion with MBP, include the monomeric Klenow polymerase and the dimeric Gene V protein of phage M13.[24][29] When the recombinant MBP includes either a defective or no signal peptide the fusion protein remains within the bacterial cytoplasm from where it can be recovered by breaking open the cells.
The fusion of proteins with MBP usually enhances their solubility and facilitates their proper folding so that the fusion proteins are most often bifunctional.[24][30] In addition, such fusions can facilitate the crystallisation of difficult proteins, e.g. membrane proteins. The crystallized protein can often have their structures solved by X-ray crystallography using molecular replacement on a known MBP structure.[31]
See also
- Protein tag
- Fluorescent glucose biosensors
- Glutathione S-transferase
References
- ↑ Duan, Xiaoqun; Hall, Jason A; Nikaido, Hiroshi; Quiocho, Florante A (March 2001). "Crystal structures of the maltodextrin/maltose-binding protein complexed with reduced oligosaccharides: flexibility of tertiary structure and ligand binding". Journal of Molecular Biology 306 (5): 1115–1126. doi:10.1006/jmbi.2001.4456. PMID 11237621.
- ↑ "Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12". The Journal of Biological Chemistry 259 (16): 10606–13. August 1984. doi:10.1016/S0021-9258(18)91005-7. PMID 6088507.
- ↑ "The 2.3-A resolution structure of the maltose- or maltodextrin-binding protein, a primary receptor of bacterial active transport and chemotaxis". The Journal of Biological Chemistry 266 (8): 5202–19. March 1991. doi:10.2210/pdb1mbp/pdb. PMID 2002054.
- ↑ "Crystallographic evidence of a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in active transport and chemotaxis". Biochemistry 31 (44): 10657–63. November 1992. doi:10.1021/bi00159a003. PMID 1420181.
- ↑ "Precursor maltose-binding protein is active in binding substrate". The Journal of Biological Chemistry 254 (20): 9979–81. October 1979. doi:10.1016/S0021-9258(19)86659-0. PMID 385604.
- ↑ "Modulation of folding pathways of exported proteins by the leader sequence". Science 239 (4843): 1033–5. February 1988. doi:10.1126/science.3278378. PMID 3278378. Bibcode: 1988Sci...239.1033P.
- ↑ "Effect of signal peptide on the stability and folding kinetics of maltose binding protein". Biochemistry 43 (12): 3608–19. March 2004. doi:10.1021/bi0360509. PMID 15035631.
- ↑ "Folding of maltose-binding protein. Evidence for the identity of the rate-determining step in vivo and in vitro". The Journal of Biological Chemistry 268 (28): 20855–62. October 1993. doi:10.1016/S0021-9258(19)36864-4. PMID 8407916.
- ↑ "Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein". European Journal of Biochemistry 47 (1): 139–49. August 1974. doi:10.1111/j.1432-1033.1974.tb03677.x. PMID 4215651.
- ↑ "Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli". Cell 46 (6): 921–8. September 1986. doi:10.1016/0092-8674(86)90074-7. PMID 3530497.
- ↑ "Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli". Nature 285 (5760): 78–81. May 1980. doi:10.1038/285078a0. PMID 6990274. Bibcode: 1980Natur.285...78B.
- ↑ "Functional implications of secondary structure analysis of wild type and mutant bacterial signal peptides". Progress in Clinical and Biological Research 63: 399–403. 1981. PMID 7312870.
- ↑ "The conformation of a signal peptide bound by Escherichia coli preprotein translocase SecA". The Journal of Biological Chemistry 280 (38): 32753–60. September 2005. doi:10.1074/jbc.M507532200. PMID 16046390.
- ↑ "Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR". Cell 131 (4): 756–69. November 2007. doi:10.1016/j.cell.2007.09.039. PMID 18022369.
- ↑ "Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation". Microbiology and Molecular Biology Reviews 62 (1): 204–29. March 1998. doi:10.1128/MMBR.62.1.204-229.1998. PMID 9529892.
- ↑ 16.0 16.1 "Promoters of the malEFG and malK-lamB operons in Escherichia coli K12". Journal of Molecular Biology 161 (4): 519–31. November 1982. doi:10.1016/0022-2836(82)90405-3. PMID 6185687.
- ↑ "Mutations in the promoter regions of the malEFG and malK-lamB operons of Escherichia coli K12". Journal of Molecular Biology 170 (4): 861–82. November 1983. doi:10.1016/s0022-2836(83)80192-2. PMID 6417341.
- ↑ "Synergistic transcription activation: a dual role for CRP in the activation of an Escherichia coli promoter depending on MalT and CRP". The EMBO Journal 19 (19): 5222–32. October 2000. doi:10.1093/emboj/19.19.5222. PMID 11013224.
- ↑ "MalT, the regulatory protein of the Escherichia coli maltose system, is an ATP-dependent transcriptional activator". The EMBO Journal 8 (3): 981–7. March 1989. doi:10.1002/j.1460-2075.1989.tb03461.x. PMID 2524384.
- ↑ "Self-association of the Escherichia coli transcription activator MalT in the presence of maltotriose and ATP". The Journal of Biological Chemistry 274 (47): 33220–6. November 1999. doi:10.1074/jbc.274.47.33220. PMID 10559195.
- ↑ "Linker mutagenesis in the gene encoding the periplasmic maltose-binding protein of E. coli". Biochimie 67 (7–8): 849–51. 1985. doi:10.1016/s0300-9084(85)80178-4. PMID 3002495.
- ↑ "Silent and functional changes in the periplasmic maltose-binding protein of Escherichia coli K12. I. Transport of maltose". Journal of Molecular Biology 194 (4): 663–73. April 1987. doi:10.1016/0022-2836(87)90243-9. PMID 2821264.
- ↑ "Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein". Vaccine 19 (7–8): 684–93. November 2000. doi:10.1016/s0264-410x(00)00267-x. PMID 11115689.
- ↑ 24.0 24.1 24.2 "Production in Escherichia coli and one-step purification of bifunctional hybrid proteins which bind maltose. Export of the Klenow polymerase into the periplasmic space". European Journal of Biochemistry 171 (3): 541–9. February 1988. doi:10.1111/j.1432-1033.1988.tb13823.x. PMID 3278900.
- ↑ "Conformational and functional properties of an undecapeptide epitope fused with the C-terminal end of the maltose binding protein". Biochemistry 36 (29): 8954–61. July 1997. doi:10.1021/bi962508d. PMID 9220983.
- ↑ "Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein". Gene 67 (1): 21–30. July 1988. doi:10.1016/0378-1119(88)90004-2. PMID 2843437.
- ↑ "Bifunctional hybrids between the variable domains of an immunoglobulin and the maltose-binding protein of Escherichia coli: production, purification and antigen binding". Protein Engineering 7 (2): 271–80. February 1994. PMID 8170930.
- ↑ "Protein fusion tags for efficient expression and purification of recombinant proteins in the periplasmic space of E. coli". 3 Biotech 6 (1): 44. June 2016. doi:10.1007/s13205-016-0397-7. PMID 28330113.
- ↑ "Export and purification of a cytoplasmic dimeric protein by fusion to the maltose-binding protein of Escherichia coli". European Journal of Biochemistry 193 (2): 325–30. October 1990. doi:10.1111/j.1432-1033.1990.tb19341.x. PMID 2226455.
- ↑ "Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused". Protein Science 8 (8): 1668–74. August 1999. doi:10.1110/ps.8.8.1668. PMID 10452611.
- ↑ "Crystal structures of MBP fusion proteins". Protein Science 25 (3): 559–71. March 2016. doi:10.1002/pro.2863. PMID 26682969.
External links
- N-Terminal Fusion of Target Protein to Maltose-Binding Protein at Michigan Technological University
- maltose-binding+protein at the US National Library of Medicine Medical Subject Headings (MeSH)
- Generic protocol for the expression and purification of recombinant proteins in Escherichia coli using a combinatorial His6-maltose binding protein fusion tag
 |

