Biology:Multituberculata
| Multituberculates | |
|---|---|

| |
| Skeleton of Catopsbaatar | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Infraclass: | †Allotheria |
| Order: | †Multituberculata Cope, 1884 |
| Suborders | |
| |
Multituberculata (commonly known as multituberculates, named for the multiple tubercles of their teeth) is an extinct order of rodent-like mammals with a fossil record spanning over 130 million years. They first appeared in the Middle Jurassic, and reached a peak diversity during the Late Cretaceous and Paleocene. They eventually declined from the mid-Paleocene onwards, disappearing from the known fossil record in the late Eocene.[1] They are the most diverse order of Mesozoic mammals with more than 200 species known, ranging from mouse-sized to beaver-sized. These species occupied a diversity of ecological niches, ranging from burrow-dwelling to squirrel-like arborealism to jerboa-like hoppers.[2][3] Multituberculates are usually placed as crown mammals outside either of the two main groups of living mammals—Theria, including placentals and marsupials, and Monotremata[4]—but usually as closer to Theria than to monotremes.[5][6] They are considered to be closely related to Euharamiyida and Gondwanatheria as part of Allotheria.
Description

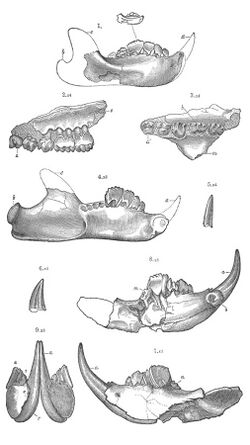
The multituberculates had a cranial and dental anatomy superficially similar to rodents such as mice and rats, with cheek-teeth separated from the chisel-like front teeth by a wide tooth-less gap (the diasteme). Each cheek-tooth displayed several rows of small cusps (or tubercles, hence the name) that operated against similar rows in the teeth of the jaw; the exact homology of these cusps to therian ones is still a matter of debate.[citation needed] Unlike rodents, which have ever-growing teeth, multituberculates underwent dental replacement patterns typical to most mammals (though in at least some species the lower incisors continued to erupt long after the root's closure).[7] Multituberculates are notable for the presence of a massive fourth lower premolar, the plagiaulacoid; other mammals, like Plesiadapiformes and diprotodontian marsupials, also have similar premolars in both upper and lower jaws, but in multituberculates this tooth is massive and the upper premolars are not modified this way. In basal multituberculates all three lower premolars were plagiaulacoids, increasing in size posteriorly, but in Cimolodonta only the fourth lower premolar remained, with the third one remaining only as a vestigial peg-like tooth,[7] and in several taxa like taeniolabidoideans, the plagiaulacoid disappeared entirely or was reconverted into a molariform tooth.[8][9][10]
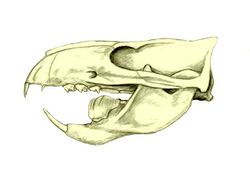
Unlike rodents and similar therians, multituberculates had a palinal jaw stroke (front-to-back), instead of a propalinal (back-to-front) or transverse (side-to-side) one; as a consequence, their jaw musculature and cusp orientation is radically different.[4][7] Palinal jaw strokes are almost entirely absent in modern mammals (with the possible exception of the dugong[11]), but are also present in haramiyidans, argyrolagoideans and tritylodontids, the former historically united with multituberculates on that basis. Multituberculate mastication is thought to have operated in a two stroke cycle: first, food held in place by the last upper premolar was sliced by the bladelike lower pre-molars as the dentary moved orthally (upward). Then the lower jaw moved palinally, grinding the food between the molar cusp rows.[4][7]
The structure of the pelvis in the Multituberculata suggests that they gave birth to tiny helpless, underdeveloped young, similar to modern marsupials, such as kangaroos.[2][7] However, a 2022 study reveals that they might actually have had long gestation periods like placentals.[12]
At least two lineages developed hypsodonty, in which tooth enamel extends beyond the gumline: lambdopsalid taeniolabidoideans[13] and sudamericid gondwanatheres.[14]
Studies published in 2018 demonstrated that multituberculates had relatively complex brains, some braincase regions even absent in therian mammals.[15]
Evolution
Multituberculates first appear in the fossil record during the Jurassic period, and then survived and even dominated for over one hundred million years, longer than any other order of mammaliforms, including placental mammals. The earliest known multituberculates are from the Middle Jurassic (Bathonian ~166-168 million years ago) of England and Russia, including Hahnotherium and Kermackodon from the Forest Marble Formation of England, and Tashtykia and Tagaria from the Itat Formation of Russia. These forms are only known from isolated teeth, which bear close similarity to those of euharamyidans, which they are suspected to be closely related.[16] During the Late Jurassic and Early Cretaceous, primitive multituberculates, collectively grouped into the paraphyletic "Plagiaulacida" were abundant and widespread across Laurasia (including Europe, Asia and North America). During the Aptian stage of the Early Cretaceous, the advanced subgroup Cimolodonta appeared in North America, characterised by a reduced number of lower premolars, with a blade-like lower fourth premolar. By the early Late Cretaceous (Cenomanian) Cimolodonta had replaced all other multituberculate lineages.[17]
During the Late Cretaceous, multituberculates experienced an adaptive radiation, corresponding with a shift towards herbivory.[18] Multituberculates reached their peak diversity during the early Paleocene, shortly after the Cretaceous–Paleogene extinction event, but declined from the mid Paleocene onwards, likely due to competition with placental mammals such as rodents and ungulates, the group finally became extinct in the Late Eocene.[19][20] There are some isolated records of multituberculates from the Southern Hemisphere, including the cimolodontan Corriebaatar from the Early Cretaceous of Australia,[21] and fragmentary remains from the Late Cretaceous Maevarano Formation of Madagascar.[22] The family Ferugliotheriidae from the Late Cretaceous of South America, traditionally considered gondwanatherians, may actually be cimolodontan multituberculates.[21]
During the Late Cretaceous and Paleocene the multituberculates radiated into a wide variety of morphotypes, including the squirrel-like arboreal ptilodonts. The peculiar shape of their last lower premolar is their most outstanding feature. These teeth were larger and more elongated than the other cheek-teeth and had an occlusive surface forming a serrated slicing blade. Though it can be assumed that this was used for crushing seeds and nuts, it is believed that most small multituberculates also supplemented their diet with insects, worms, and fruits.[4] Tooth marks attributed to multituberculates are known on Champsosaurus fossils, indicating that at least some of these mammals were scavengers.[23] A ptilodont that thrived in North America was Ptilodus. Thanks to the well-preserved Ptilodus specimens found in the Bighorn Basin, Wyoming, we know that these multituberculates were able to abduct and adduct their big toes, and thus that their foot mobility was similar to that of modern squirrels, which descend trees head first.[4]

Another group of multituberculates, the taeniolabids, were heavier and more massively built, indicating that they lived a fully terrestrial life. The largest specimens weighed probably as much as 22 kg (49 lb), making them comparable in size to large rodents like the modern beaver.[24][25]
Classification
Multituberculate is generally placed in the Allotheria alongside Euharamiyida, a clade of mammals known from the Middle Jurassic to Early Cretaceous of the Asia and possibly Europe that several morphological similarities to multituberculates.[16][26]
Gondwanatheria is a monophyletic group of allotherians that was diverse in the Late Cretaceous of South America, India, Madagascar and possibly Africa and occurs onwards into the Paleogene of South America and Antarctica. Their placement within Allotheria is highly controversial, with some phylogenies recovering the group as deeply nested within multituberculates, while others recover them as a distinct branch of allotherians separate from multituberculates.[26]

In their 2001 study, Kielan-Jaworowska and Hurum found that most multituberculates could be referred to two suborders: "Plagiaulacida" and Cimolodonta. The exception is the genus Arginbaatar, which shares characteristics with both groups.
"Plagiaulacida" is paraphyletic, representing the more primitive evolutionary grade. Its members are the more basal Multituberculata. Chronologically, they ranged from perhaps the Middle Jurassic until the mid-Cretaceous. This group is further subdivided into three informal groupings: the allodontid line, the paulchoffatiid line, and the plagiaulacid line.
Cimolodonta is, apparently, a natural (monophyletic) suborder. This includes the more derived Multituberculata, which have been identified from the lower Cretaceous to the Eocene. The superfamilies Djadochtatherioidea, Taeniolabidoidea, Ptilodontoidea are recognized, as is the Paracimexomys group. Additionally, there are the families Cimolomyidae, Boffiidae, Eucosmodontidae, Kogaionidae, Microcosmodontidae and the two genera Uzbekbaatar and Viridomys. More precise placement of these types awaits further discoveries and analysis.[27][better source needed]
Taxonomy
Subgroups
| ||||
|---|---|---|---|---|
 Based on the combined works of Mikko's Phylogeny Archive[29] and Paleofile.com.[citation needed] Suborder †Plagiaulacida Simpson 1925
|
Phylogeny[28]
| Multituberculata |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Paleoecology
Behaviour
Multituberculates are some of the earliest mammals to display complex social behaviours. One species, Filikomys, from the Late Cretaceous of North America, engaged in multi-generational group nesting and burrowing.[30]
Extinction
The extinction of multituberculates has been a topic of controversy for several decades.[31] After at least 88 million years of dominance over most mammalian assemblies, multituberculates reached the peak of their diversity in the early Palaeocene, before gradually declining across the final stages of the epoch and the Eocene, finally disappearing in the early Oligocene.[32]
The last multituberculate species, Ectypodus childei, went extinct near the end of the Eocene in North America. It is unclear why this particular species persisted for so long when all of its counterparts succumbed to replacement by rodents.[33]: 43
Traditionally, the extinction of multituberculates has been linked to the rise of rodents (and, to a lesser degree, earlier placental competitors like hyopsodonts and Plesiadapiformes), which supposedly competitively excluded multituberculates from most mammalian faunas.[1]
However, the idea that multituberculates were replaced by rodents and other placentals has been criticised by several authors. For one thing, it relies on the assumption that these mammals are "inferior" to more derived placentals, and ignores the fact that rodents and multituberculates had co-existed for at least 15 million years. According to some researchers, multituberculate "decline" is shaped by sharp extinction events, most notably after the Tiffanian, where a sudden drop in diversity occurs. Finally, the youngest known multituberculates do not exemplify patterns of competitive exclusion; the Oligocene Ectypodus is a rather generalistic species, rather than a specialist. This combination of factors suggests that, rather than gradually declining due to pressure from rodents and similar placentals, multituberculates simply could not cope with climatic and vegetation changes, as well as the rise of new predatory eutherians, such as miacids.[32]
More recent studies show a mixed effect. Multituberculate faunas in North America and Europe do indeed decline in correlation to the introduction of rodents in these areas. However, Asian multituberculate faunas co-existed with rodents with minimal extinction events, implying that competition was not the main cause for the extinction of Asiatic multituberculates. As a whole, it seems that Asian multituberculates, unlike North American and European species, never recovered from the KT event, which allowed the evolution and propagation of rodents in the first place.[31] A recent study seems to indeed indicate that eutherians recovered more quickly from the KT event than multituberculates.[34] Conversely, another study has shown that placental radiation did not start significantly until after the decline of multituberculates.[20]
References
- ↑ 1.0 1.1 Krause, David W. (1986). "Competitive exclusion and taxonomic displacement in the fossil record". Vertebrates, Phylogeny, and Philosophy. pp. 95–117. doi:10.2113/gsrocky.24.special_paper_3.95. ISBN 978-0-941570-02-2. https://archive.org/details/vertebratesphylo0000unse/page/95.
- ↑ 2.0 2.1 Weil, Anne (June 1997). "Introduction to Multituberculates: The 'Lost Tribe' of Mammals". Berkeley: UCMP. http://www.ucmp.berkeley.edu/mammal/multis/multis.html.
- ↑ Chen, Meng; Philip Wilson, Gregory (2015). "A multivariate approach to infer locomotor modes in Mesozoic mammals". Paleobiology 41 (2): 280–312. doi:10.1017/pab.2014.14. Bibcode: 2015Pbio...41..280C.
- ↑ 4.0 4.1 4.2 4.3 4.4 Agustí-Antón 2002, pp 3-4
- ↑ Benton, Michael J. Vertebrate Palaeontology (2004), p. 300
- ↑ Carrano, Matthew T., and Richard W. Blob, Timothy J. Gaudin, and John R. Wible (2006). Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles, p. 358.
- ↑ 7.0 7.1 7.2 7.3 7.4 Kielan-Jaworowska, Zofia, Richard L. Cifelli, and Zhe-Xi Luo (2005). Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure , p. 299
- ↑ Gurovich 2005 p. 334[full citation needed]
- ↑ Gurovich, Yamila; Beck, Robin (March 2009). "The Phylogenetic Affinities of the Enigmatic Mammalian Clade Gondwanatheria". Journal of Mammalian Evolution 16 (1): 25–49. doi:10.1007/s10914-008-9097-3.
- ↑ Rougier et al. 2009 p.233[full citation needed]
- ↑ Lanyon, J. M.; Sanson, G. D. (February 2006). "Degenerate dentition of the dugong (Dugong dugon), or why a grazer does not need teeth: morphology, occlusion and wear of mouthparts". Journal of Zoology 268 (2): 133–152. doi:10.1111/j.1469-7998.2005.00004.x.
- ↑ "New study challenges old views on what's 'primitive' in mammalian reproduction". 25 July 2022. https://news.umich.edu/new-study-challenges-old-views-on-whats-primitive-in-mammalian-reproduction/.
- ↑ Williamson, Thomas E.; Brusatte, Stephen L.; Secord, Ross; Shelley, Sarah (2015). "A new taeniolabidoid multituberculate (Mammalia) from the middle Puercan of the Nacimiento Formation, New Mexico, and a revision of taeniolabidoid systematics and phylogeny". Zoological Journal of the Linnean Society 177: 183–208. doi:10.1111/zoj.12336.
- ↑ "Gondwanatheria". https://books.google.com/books?id=JgKGv2aQxggC&q=gondwanatheria+grass&pg=PA32.[|permanent dead link|dead link}}]
- ↑ Crompton, A. W.; Musinsky, C.; Rougier, G. W.; Bhullar, B.-A. S.; Miyamae, J. A. (September 2018). "Origin of the Lateral Wall of the Mammalian Skull: Fossils, Monotremes and Therians Revisited". Journal of Mammalian Evolution 25 (3): 301–313. doi:10.1007/s10914-017-9388-7.
- ↑ 16.0 16.1 Averianov, Alexander O.; Martin, Thomas; Lopatin, Alexey V.; Schultz, Julia A.; Schellhorn, Rico; Krasnolutskii, Sergei; Skutschas, Pavel; Ivantsov, Stepan (May 2021). "Multituberculate mammals from the Middle Jurassic of Western Siberia, Russia, and the origin of Multituberculata". Papers in Palaeontology 7 (2): 769–787. doi:10.1002/spp2.1317. ISSN 2056-2799. https://zenodo.org/record/3877503.
- ↑ Weaver, Lucas N.; Wilson, Gregory P.; Krumenacker, L. J.; Mclaughlin, Kayla; Moore, Jason R.; Varricchio, David J. (2019-03-04). "New multituberculate mammals from the mid-Cretaceous (lower Cenomanian) Wayan Formation of southeastern Idaho and implications for the early evolution of Cimolodonta" (in en). Journal of Vertebrate Paleontology 39 (2): e1604532. doi:10.1080/02724634.2019.1604532. ISSN 0272-4634. Bibcode: 2019JVPal..39E4532W. https://www.tandfonline.com/doi/full/10.1080/02724634.2019.1604532.
- ↑ Wilson, Gregory P.; Evans, Alistair R.; Corfe, Ian J.; Smits, Peter D.; Fortelius, Mikael; Jernvall, Jukka (March 2012). "Adaptive radiation of multituberculate mammals before the extinction of dinosaurs" (in en). Nature 483 (7390): 457–460. doi:10.1038/nature10880. ISSN 1476-4687. PMID 22419156. Bibcode: 2012Natur.483..457W. https://www.nature.com/articles/nature10880.
- ↑ Adams, Neil F.; Rayfield, Emily J.; Cox, Philip G.; Cobb, Samuel N.; Corfe, Ian J. (March 2019). "Functional tests of the competitive exclusion hypothesis for multituberculate extinction" (in en). Royal Society Open Science 6 (3): 181536. doi:10.1098/rsos.181536. ISSN 2054-5703. PMID 31032010. Bibcode: 2019RSOS....681536A.
- ↑ 20.0 20.1 Brocklehurst, Neil; Panciroli, Elsa; Benevento, Gemma Louise; Benson, Roger B. J. (July 2021). "Mammaliaform extinctions as a driver of the morphological radiation of Cenozoic mammals". Current Biology 31 (13): 2955–2963.e4. doi:10.1016/j.cub.2021.04.044. PMID 34004143. https://ora.ox.ac.uk/objects/uuid:bda82407-db76-4c15-b061-ceb9ae271dd5.
- ↑ 21.0 21.1 Rich, Thomas; Trusler, Peter; Kool, Lesley; White, Matt A.; Bevitt, Joseph; Morton, Steven; Vickers−Rich, Patricia (2022). "Second specimen of Corriebaatar marywaltersae from the Lower Cretaceous of Australia confirms its multituberculate affinities". Acta Palaeontologica Polonica 67. doi:10.4202/app.00924.2021. ISSN 0567-7920.
- ↑ Krause, David W.; Hoffmann, Simone; Werning, Sarah (December 2017). "First postcranial remains of Multituberculata (Allotheria, Mammalia) from Gondwana" (in en). Cretaceous Research 80: 91–100. doi:10.1016/j.cretres.2017.08.009. Bibcode: 2017CrRes..80...91K.
- ↑ Longrich, Nicholas R.; Ryan, Michael J. (2010). "Mammalian tooth marks on the bones of dinosaurs and other Late Cretaceous vertebrates". Palaeontology 53 (4): 703–709. doi:10.1111/j.1475-4983.2010.00957.x. Bibcode: 2010Palgy..53..703L.
- ↑ Krause et al 2021
- ↑ Wilson et al 2012
- ↑ 26.0 26.1 Hoffmann, Simone; Beck, Robin M. D.; Wible, John R.; Rougier, Guillermo W.; Krause, David W. (2020-12-14). "Phylogenetic placement of Adalatherium hui (Mammalia, Gondwanatheria) from the Late Cretaceous of Madagascar: implications for allotherian relationships" (in en). Journal of Vertebrate Paleontology 40 (sup1): 213–234. doi:10.1080/02724634.2020.1801706. ISSN 0272-4634. Bibcode: 2020JVPal..40S.213H. https://www.tandfonline.com/doi/full/10.1080/02724634.2020.1801706.
- ↑ Dykes Multituberculata (Cope 1884)
- ↑ 28.0 28.1 Nicolás R. Chimento; Federico L. Agnolin; Fernando E. Novas (2015). "The bizarre 'metatherians' Groeberia and Patagonia, late surviving members of gondwanatherian mammals". Historical Biology: An International Journal of Paleobiology 27 (5): 603–623. doi:10.1080/08912963.2014.903945.
- ↑ Mikko's Phylogeny Archive Haaramo, Mikko (2007). "Mammaliaformes – mammals and near-mammals". http://www.helsinki.fi/~mhaaramo/metazoa/deuterostoma/chordata/synapsida/basal_mammalia/mammaliaformes_1.html.
- ↑ Weaver, Lucas N.; Varricchio, David J.; Sargis, Eric J.; Chen, Meng; Freimuth, William J.; Wilson Mantilla, Gregory P. (2 November 2020). "Early mammalian social behaviour revealed by multituberculates from a dinosaur nesting site". Nature Ecology & Evolution 5 (1): 32–37. doi:10.1038/s41559-020-01325-8. PMID 33139921.
- ↑ 31.0 31.1 Wood, D. Joseph (2010). The Extinction of the Multituberculates Outside North America: a Global Approach to Testing the Competition Model (M.S.). The Ohio State University. Archived from the original on 2015-04-08. Retrieved 2015-04-03.
- ↑ 32.0 32.1 Ostrander, Gregg (1 January 1984). "The Early Oligocene (Chadronian) Raben Ranch Local Fauna, Northwest Nebraska: Multituberculata; with Comments on the Extinction of the Allotheria". Transactions of the Nebraska Academy of Sciences and Affiliated Societies. https://digitalcommons.unl.edu/tnas/239/.
- ↑ Wood, D. Joseph (2010). The Extinction of the Multituberculates Outside North America: a Global Approach to Testing the Competition Model (Thesis). The Ohio State University. Archived from the original on 2023-05-19. Retrieved 2023-05-19.
- ↑ Pires, Mathias M.; Rankin, Brian D.; Silvestro, Daniele; Quental, Tiago B. (1804). "Diversification dynamics of mammalian clades during the K–Pg mass extinction". Biology Letters 14 (9): 2058. doi:10.1098/rsbl.2018.0458. PMID 30258031.
Sources
- Agustí, Jordi; Antón, Mauricio (2002). Mammoths, Sabertooths, and Hominids: 65 Millions Years of Mammalian Evolution in Europe. New York: Columbia University Press. ISBN 978-0-231-11640-4.
- Dykes, Trevor. "Multituberculata (Cope 1884)". http://home.arcor.de/ktdykes/multis.htm.
- Kielan-Jaworowska, Zofia; Hurum, Jørn H. (2001). "Phylogeny and Systematics of multituberculate mammals". Palaeontology 44 (3): 389–429. doi:10.1111/1475-4983.00185. Bibcode: 2001Palgy..44..389K. http://doc.rero.ch/record/14775/files/PAL_E1903.pdf.
Wikidata ☰ Q131181 entry
 |
