Biology:Mycobacterium
| Mycobacterium | |
|---|---|
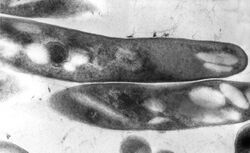
| |
| TEM micrograph of M. tuberculosis | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Mycobacteriales |
| Family: | Mycobacteriaceae |
| Genus: | Mycobacterium Lehmann & Neumann 1896[1] |
| Species | |
|
Over 190 species, see LPSN | |
| Synonyms | |
| |
Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis (M. tuberculosis) and leprosy (M. leprae) in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces.[2] Since this genus has cell walls with a waxy lipid-rich outer layer that contains high concentrations of mycolic acid,[3] acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.[4]
Mycobacterial species are generally aerobic, non-motile, and capable of growing with minimal nutrients. The genus is divided based on each species' pigment production and growth rate.[5] While most Mycobacterium species are non-pathogenic, the genus' characteristic complex cell wall contributes to evasion from host defenses.[6]
Microbiology
Morphology

Mycobacteria are aerobic with 0.2-0.6 µm wide and 1.0-10 µm long rod shapes. They are generally non-motile, except for the species Mycobacterium marinum, which has been shown to be motile within macrophages.[7] Mycobacteria possess capsules and most do not form endospores. M. marinum and perhaps M. bovis have been shown to sporulate;[8] however, this has been contested by further research.[9] The distinguishing characteristic of all Mycobacterium species is a thick, hydrophobic, and mycolic acid-rich cell wall made of peptidoglycan and arabinogalactan, with these unique components offering targets for new tuberculosis drugs.[10]
Physiology
Many Mycobacterium species readily grow with minimal nutrients, using ammonia and/or amino acids as nitrogen sources and glycerol as a carbon source in the presence of mineral salts. Temperatures for optimal growth vary between species and media conditions, ranging from 25 to 45 °C.[5]
Most Mycobacterium species, including most clinically relevant species, can be cultured in blood agar.[11] However, some species grow very slowly due to extremely long reproductive cycles, such as M. leprae requiring 12 days per division cycle compared to 20 minutes for some E. coli strains.[12]
Ecology
Whereas Mycobacterium tuberculosis and M. leprae are pathogenic, most mycobacteria do not cause disease unless they enter skin lesions of those with pulmonary and/or immune dysfunction, despite being widespread across aquatic and terrestrial environments. Through biofilm formation, cell wall resistance to chlorine, and association with amoebas, mycobacteria can survive a variety of environmental stressors. The agar media used for most water testing does not support the growth of mycobacteria, allowing it to go undetected in municipal and hospital systems.[13]
Genomics
Hundreds of Mycobacterium genomes have been completely sequenced.[14]
The genome sizes of mycobacteria range from relatively small ones (e.g. in M. leprae) to quite large ones, such as that as M. vulneris, encoding 6,653 proteins, larger than the ~6000 proteins of eukaryotic yeast.[15]
| Organism | Number of Protein Coding Genes |
|---|---|
| M. intracellulare | 5,289[16] |
| M. colombiense | 5,084[17] |
| M. leprae | 1,603[18] |
| M. tuberculosis | 3,995[18] |
| M. smegmatis | 6,602[19] |
| M. chelonae | 4,948[20] |
Pathogenicity
Mycobacterium tuberculosis complex
Mycobacterium tuberculosis can remain latent in human hosts for decades after an initial infection, allowing it to continue infecting others. It has been estimated that a third of the world population has latent tuberculosis (TB).[21] M. tuberculosis has many virulence factors, which can be divided across lipid and fatty acid metabolism, cell envelope proteins, macrophage inhibitors, kinase proteins, proteases, metal-transporter proteins, and gene expression regulators.[22] Several lineages such as M. t. var. bovis (bovine TB) were considered separate species in the M, tuberculosis complex until they were finally merged into the main species in 2018.[23]
Leprosy
The development of Leprosy is caused by infection with either Mycobacterium leprae or Mycobacterium lepromatosis, two closely related bacteria. Roughly 200,000 new cases of infection are reported each year, and 80% of new cases are reported in Brazil, India, and Indonesia.[24] M. leprae infection localizes within the skin macrophages and Schwann cells found in peripheral nerve tissue.
Nontuberculosis Mycobacteria

Nontuberculosis Mycobacteria (NTM), which exclude M. tuberculosis, M. leprae, and M. lepromatosis, can infect mammalian hosts. These bacteria are referred to as "atypical mycobacteria." Although person-to-person transmission is rare, transmission of M. abscessus has been observed between patients with cystic fibrosis.[25] The four primary diseases observed in humans are chronic pulmonary disease, disseminated disease in immunocompromised patients, skin and soft tissue infections, and superficial lymphadenitis. 80-90% of recorded NTM infections manifest as pulmonary diseases.[26]
M. abscessus is the most virulent rapidly-growing mycobacterium (RGM), as well as the leading cause of RGM based pulmonary infections. Although it has been traditionally viewed as an opportunistic pathogen like other NTMs, analysis of various virulence factors (VFs) have shifted this view to that of a true pathogen. This is due to the presence of known mycobacterial VFs and other non-mycobacterial VFs found in other prokaryotic pathogens.[26]
Virulence factors
Mycobacteria have cell walls with peptidoglycan, arabinogalactan, and mycolic acid; a waxy outer mycomembrane of mycolic acid; and an outermost capsule of glucans and secreted proteins for virulence. It constantly remodels these layers to survive in stressful environments and avoid host immune defenses. This cell wall structure results in colony surfaces resembling fungi, leading to the genus' use of the Greek prefix myco-.[27] This unique structure makes penicillins ineffective, instead requiring a multi-drug antibiotic treatment of isoniazid to inhibit mycolic acid synthesis, rifampicin to interfere with transcription, ethambutol to hinder arabinogalactan synthesis, and pyrazinamide to impede Coenzyme A synthesis.[6]
| Organism | Common Symptoms of Infection | Known Treatments | Reported Cases (Region, Year) |
|---|---|---|---|
| M. tuberculosis | Fatigue, weight loss, fever, hemoptysis, chest pain.[28] | isoniazid INH, rifampin, pyrazinamide, ethambutol.[29] | 1.6 Million (Global, 2021)[30] |
| M. leprae
M. lepromatosis |
Skin discoloration, nodule development, dry skin, loss of eyebrows and/or eyelashes, numbness, nosebleeds, paralysis, blindness, nerve pain.[31] | dapson, rifampicin, clofazimine.[31] | 133,802 (Global, 2021)[32] |
| M. avium complex | Tender skin, development of boils or pus-filled vesicles, fevers, chills, muscle aches.[33] | clarithromycin, azithromycin, amikacin, cefoxitin, imipenem.[34] | 3000 (US, Annual estimated)[35] |
| M. abscessus complex | Coughing, hemoptysis, fever, cavitary lesions.[36] | clarithromycin, amikacin, cefoxitin, imipenem.[36] | Unknown |
History
| Cladogram of Key Species | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mycobacteria have historically been categorized through phenotypic testing, such as the Runyon classification of analyzing growth rate and production of yellow/orange carotenoid pigments. Group I contains photochromogens (pigment production induced by light), Group II comprises scotochromogens (constitutive pigment production), and the non-chromogens of Groups III and IV have a pale yellow/tan pigment, regardless of light exposure. Group IV species are "rapidly-growing" mycobacteria compared to the "slowly-growing" Group III species because samples grow into visible colonies in less than seven days.[5]
Because the International Code of Nomenclature of Prokaryotes (ICNP) currently recognizes 195 Mycobacterium species, classification and identification systems now rely on DNA sequencing and computational phylogenetics. The major disease-causing groups are the M. tuberculosis complex (tuberculosis), M. avium complex (mycobacterium avium-intracellulare infection), M. leprae and M. lepromatosis (leprosy), and M. abscessus (chronic lung infection).[2]
Microbiologist Enrico Tortoli has constructed a phylogenetic tree of the genus' key species based on the earlier genetic sequencing of Rogall, et al. (1990), alongside new phylogentic trees based on Tortoli's 2017 sequencing of 148 Mycobacterium species:[37]


Proposed division of the genus
Gupta et al. have proposed dividing Mycobacterium into five genera, based on an analysis of 150 species in this genus. Due to controversy over complicating clinical diagnoses and treatment, all of the renamed species have retained their original identity in the Mycobacterium genus as a valid taxonomic synonym:[39][40]
- Mycobacterium based on the Slowly-Growing Tuberculosis-Simiae clade
- Mycobacteroides based on the Rapidly-Growing Abscessus-Chelonae clade
- Mycolicibacillus based on the Slowly-Growing Triviale clade
- Mycolicibacter based on the Slowly-Growing Terrae clade
- Mycolicibacterium based on the Rapidly-Growing Fortuitum-Vaccae clade
Diagnosis
The two most common methods for visualizing these acid-fast bacilli as bright red against a blue background are the Ziehl-Neelsen stain and modified Kinyoun stain. Fite's stain is used to color M. leprae cells as pink against a blue background. Rapid Modified Auramine O Fluorescent staining has specific binding to slowly-growing mycobacteria for yellow staining against a dark background. Newer methods include Gomori-Methenamine Silver staining and Perioidic Acid Schiff staining to color Mycobacterium avium complex (MAC) cells black and pink, respectively.[4]
While some mycobacteria can take up to eight weeks to grow visible colonies from a cultured sample, most clinically relevant species will grow within the first four weeks, allowing physicians to consider alternative causes if negative readings continue past the first month.[41] Growth media include Löwenstein–Jensen medium and mycobacteria growth indicator tube (MGIT).
-
Mycobacterium tuberculosis on Ziehl-Neelsen stain
-
Slant tubes of Löwenstein-Jensen medium.[note 1]
-
MGIT samples emitting fluorescence in ultraviolet light
Mycobacteriophages
Mycobacteria can be infected by mycobacteriophages, a class of viruses with high specificity for their targets. By hijacking the cellular machinery of mycobacteria to produce additional phages, such viruses can be used in phage therapy for eukaryotic hosts, as they would die alongside the mycobacteria. Since only some mycobacteriophages are capable of penetrating the M. tuberculosis membrane, the viral DNA may be delivered through artificial liposomes because bacteria uptake, transcribe, and translate foreign DNA into proteins.[42]
Mycosides
Mycosides are glycolipids isolated from Mycobacterium species with Mycoside A found in photochromogenic strains, Mycoside B in bovine strains, and Mycoside C in avian strains.[43] Different forms of Mycoside C have varying success as a receptor to inactivate mycobacteriophages.[44] Replacement of the gene encoding mycocerosic acid synthase in M. bovis prevents formation of mycosides.[45]
Notes
- ↑ From left to right in image of slant tubes of Löwenstein-Jensen medium:
- Negative control
- M. tuberculosis: Dry-appearing colonies
- Mycobacterium avium complex: Wet-appearing colonies
- M. gordonae: Yellowish colonies
References
- ↑ Atlas und Grundriss der Bakteriologie und Lehrbuch der speziellen bakteriologischen Diagnostik (1st ed.). München: J.F. Lehmann. 1896.
- ↑ 2.0 2.1 "Mycobacteria: Health Advisory". Environmental Protection Agency. August 1999. https://www.epa.gov/sites/default/files/2015-10/documents/mycobacteria-report.pdf.
- ↑ "The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host's immune system". The Biochemical Journal 477 (10): 1983–2006. May 2020. doi:10.1042/BCJ20200194. PMID 32470138.
- ↑ 4.0 4.1 "Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease". Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 24: 100244. August 2021. doi:10.1016/j.jctube.2021.100244. PMID 34036184.
- ↑ 5.0 5.1 5.2 "Practical Guidance for Clinical Microbiology Laboratories: Mycobacteria". Clinical Microbiology Reviews 31 (2): e00038–17. April 2018. doi:10.1128/CMR.00038-17. PMID 29386234.
- ↑ 6.0 6.1 "The mycobacterial cell envelope - a moving target". Nature Reviews. Microbiology 18 (1): 47–59. January 2020. doi:10.1038/s41579-019-0273-7. PMID 31728063.
- ↑ "Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility". The Journal of Experimental Medicine 198 (9): 1361–1368. November 2003. doi:10.1084/jem.20031072. PMID 14597736.
- ↑ "Sporulation in mycobacteria". Proceedings of the National Academy of Sciences of the United States of America 106 (26): 10781–10786. June 2009. doi:10.1073/pnas.0904104106. PMID 19541637. Bibcode: 2009PNAS..10610781G.
- ↑ "Do mycobacteria produce endospores?". Proceedings of the National Academy of Sciences of the United States of America 107 (2): 878–881. January 2010. doi:10.1073/pnas.0911299107. PMID 20080769. Bibcode: 2010PNAS..107..878T.
- ↑ "Mycobacterial Cell Wall Arabinogalactan". Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. 2009. ISBN 978-1-904455-45-5.
- ↑ "Current and past strategies for bacterial culture in clinical microbiology". Clinical Microbiology Reviews 28 (1): 208–236. January 2015. doi:10.1128/CMR.00110-14. PMID 25567228.
- ↑ "Mycobacterium leprae in Mice: Minimal Infectious Dose, Relationship Between Staining Quality and Infectivity, and Effect of Cortisone". Journal of Bacteriology 89 (2): 365–372. February 1965. doi:10.1128/jb.89.2.365-372.1965. PMID 14255702.
- ↑ "Mycobacteria in drinking water distribution systems: ecology and significance for human health". FEMS Microbiology Reviews 29 (5): 911–934. November 2005. doi:10.1016/j.femsre.2005.02.001. PMID 16219512.
- ↑ "JGI GOLD | Projects". https://gold.jgi.doe.gov/projects?Project.Is+Public=Yes&Organism.Organism+Name=Mycobacterium&Organism.Organism+Name=Mycobacterium&Project.Project+Status=Complete+and+Published&Project.Is+Public=Yes&Project.Project+Status=Complete+and+Published.
- ↑ "Draft Genome Sequence of Mycobacterium vulneris DSM 45247T". Genome Announcements 2 (3). May 2014. doi:10.1128/genomeA.00370-14. PMID 24812218.
- ↑ "UniProt". https://www.uniprot.org/proteomes/UP000595205.
- ↑ "UniProt". https://www.uniprot.org/proteomes/UP000250915.
- ↑ 18.0 18.1 "UniProt". https://www.uniprot.org/proteomes/UP000000806.
- ↑ "UniProt". https://www.uniprot.org/proteomes/UP000000757.
- ↑ "UniProt". https://www.uniprot.org/proteomes/UP000317728.
- ↑ "Latent Mycobacterium tuberculosis infection". The New England Journal of Medicine 372 (22): 2127–2135. May 2015. doi:10.1056/NEJMra1405427. PMID 26017823.
- ↑ "Virulence factors of the Mycobacterium tuberculosis complex". Virulence 4 (1): 3–66. January 2013. doi:10.4161/viru.22329. PMID 23076359.
- ↑ Riojas, Marco A.; McGough, Katya J.; Rider-Riojas, Cristin J.; Rastogi, Nalin; Hazbón, Manzour Hernando (1 January 2018). "Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis". International Journal of Systematic and Evolutionary Microbiology 68 (1): 324–332. doi:10.1099/ijsem.0.002507. PMID 29205127.
- ↑ "Pathogenicity and virulence of Mycobacterium leprae". Virulence 13 (1): 1985–2011. December 2022. doi:10.1080/21505594.2022.2141987. PMID 36326715.
- ↑ "Nontuberculous Mycobacteria (NTM) Infections | HAI | CDC" (in en-us). 2019-08-12. https://www.cdc.gov/hai/organisms/nontuberculous-mycobacteria.html.
- ↑ 26.0 26.1 "General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus". Journal of Clinical Medicine 9 (8): 2541. August 2020. doi:10.3390/jcm9082541. PMID 32781595.
- ↑ "Mycobacteria: Health Advisory EPA-822-B-01-007". US Environmental Protection Agency (EPA). August 1999. p. 2. https://www.epa.gov/sites/default/files/2015-10/documents/mycobacteria-report.pdf.
- ↑ "Fact Sheets | General | Tuberculosis: General Information | TB | CDC" (in en-us). 2022-08-17. https://www.cdc.gov/tb/publications/factsheets/general/tb.htm.
- ↑ "Diagnosing and Treating Tuberculosis" (in en). American Lung Association. https://www.lung.org/lung-health-diseases/lung-disease-lookup/tuberculosis/treating-and-managing.
- ↑ "Global Tuberculosis Report 2022" (in en). https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
- ↑ 31.0 31.1 "Signs and Symptoms | Hansen's Disease (Leprosy) | CDC" (in en-us). 2018-10-22. https://www.cdc.gov/leprosy/symptoms/index.html.
- ↑ "Global leprosy (Hansen disease) update, 2021: moving towards interruption of transmission" (in en). https://www.who.int/publications-detail-redirect/who-wer9736-429-450.
- ↑ "Mycobacterium abscessus in Healthcare Settings | HAI | CDC" (in en-us). 2021-11-15. https://www.cdc.gov/hai/organisms/mycobacterium.html.
- ↑ "Treatment for Mycobacterium abscessus complex-lung disease". Journal of the Formosan Medical Association = Taiwan Yi Zhi. Consensus Statement of Nontuberculous Mycobacterial Lung Disease in Taiwan 119 (Suppl 1): S58–S66. June 2020. doi:10.1016/j.jfma.2020.05.028. PMID 32527504.
- ↑ "Mycobacterium Avium-Intracellulare Infections" (in en), xPharm: The Comprehensive Pharmacology Reference (New York: Elsevier): pp. 1–5, 2007-01-01, doi:10.1016/b978-008055232-3.60891-8, ISBN 978-0-08-055232-3, https://www.sciencedirect.com/science/article/pii/B9780080552323608918, retrieved 2023-05-07
- ↑ 36.0 36.1 "Clinical outcome of Mycobacterium abscessus infection and antimicrobial susceptibility testing". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi 43 (5): 401–406. October 2010. doi:10.1016/S1684-1182(10)60063-1. PMID 21075707.
- ↑ "Chapter 1 - The Taxonomy of the Genus Mycobacterium" (in en). Nontuberculous Mycobacteria (NTM). Academic Press. 2019-01-10. pp. 1–10. doi:10.1016/B978-0-12-814692-7.00001-2. ISBN 978-0-12-814692-7.
- ↑ "The new phylogeny of the genus Mycobacterium: The old and the news". Infection, Genetics and Evolution 56: 19–25. December 2017. doi:10.1016/j.meegid.2017.10.013. PMID 29030295.
- ↑ "Phylogenomics and Comparative Genomic Studies Robustly Support Division of the Genus Mycobacterium into an Emended Genus Mycobacterium and Four Novel Genera". Frontiers in Microbiology 9: 67. 2018. doi:10.3389/fmicb.2018.00067. PMID 29497402.
- ↑ "Same meat, different gravy: ignore the new names of mycobacteria". The European Respiratory Journal 54 (1): 1900795. July 2019. doi:10.1183/13993003.00795-2019. PMID 31296783.
- ↑ "Incubation time of Mycobacterium tuberculosis complex sputum cultures in BACTEC MGIT 960: 4weeks of negative culture is enough for physicians to consider alternative diagnoses". Diagnostic Microbiology and Infectious Disease 83 (2): 162–164. October 2015. doi:10.1016/j.diagmicrobio.2015.07.002. PMID 26239846.
- ↑ "Phage therapy as a renewed therapeutic approach to mycobacterial infections: a comprehensive review" (in English). Infection and Drug Resistance 12: 2943–2959. 2019-09-17. doi:10.2147/IDR.S218638. PMID 31571947.
- ↑ "Mycosides: a new class of type-specific glycolipids of Mycobacteria". Nature 186 (4728): 887–888. June 1960. doi:10.1038/186887a0. PMID 13831939. Bibcode: 1960Natur.186..887S.
- ↑ "Mycosides C: behavior as receptor site substance for mycobacteriophage D4". Journal of Virology 9 (6): 999–1003. June 1972. doi:10.1128/jvi.9.6.999-1003.1972. PMID 4113889.
- ↑ "Targeted replacement of the mycocerosic acid synthase gene in Mycobacterium bovis BCG produces a mutant that lacks mycosides". Proceedings of the National Academy of Sciences of the United States of America 93 (10): 4787–4792. May 1996. doi:10.1073/pnas.93.10.4787. PMID 8643481. Bibcode: 1996PNAS...93.4787A.
External links
- Bacterial and Viral Bioinformatics Resource Center: Genomes, proteins, epitopes, and pathways of mycobacteria
- Merck Manual - Mycobacteria
- Mycobrowser: Genomic and proteomic database for pathogenic mycobacteria
- CDC - Nontuberculous Mycobacteria (NTM) Infections
- PRASITE: Identification of Mycobacteria
- TB Structural Genomics Consortium
Wikidata ☰ Q194309 entry
 |

![Slant tubes of Löwenstein-Jensen medium.[note 1]](/wiki/images/thumb/4/4a/Slant_tubes_of_L%C3%B6wenstein-Jensen_medium_with_control%2C_M_tuberculosis%2C_M_avium_and_M_gordonae.jpg/250px-Slant_tubes_of_L%C3%B6wenstein-Jensen_medium_with_control%2C_M_tuberculosis%2C_M_avium_and_M_gordonae.jpg)

