Chemistry:Glucan
A glucan is a polysaccharide derived from D-glucose,[1] linked by glycosidic bonds. Glucans are noted in two forms: alpha glucans and beta glucans. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.
In the field of bacteriology, the term polyglucan is used to describe high molecular mass glucans. They are structural polysaccharide consisting of a long linear chain of several hundred to many thousands D-glucose monomers.[2] The point of attachment is O-glycosidic bonds, where a glycosidic oxygen links the glycoside to the reducing end sugar. Polyglucans naturally occur in the cell walls of bacteria. Bacteria produce this polysaccharide in a cluster near the bacteria's cells. Polyglucan's are a source of beta-glucans. Structurally, beta 1.3-glucans are complex glucose homopolymers binding together in a beta-1,3 configuration.[3]
Types
The following are glucans (The α- and β- and numbers clarify the type of O-glycosidic bond and the specific carbons involved):[4]
Alpha
- dextran, α-1,6-glucan with α-1,3-branches
- floridean starch, α-1,4- and α-1,6-glucan
- glycogen, α-1,4- and α-1,6-glucan
- pullulan, α-1,4- and α-1,6-glucan
- starch, a mixture of amylose and amylopectin, both α-1,4- and α-1,6-glucans
- α-1,2-glucan, α-1,2-glucan
Beta
- cellulose, β-1,4-glucan
- chrysolaminarin, β-1,3-glucan
- curdlan, β-1,3-glucan
- laminarin, β-1,3- and β-1,6-glucan
- lentinan, a strictly purified β-1,6:β-1,3-glucan from Lentinus edodes
- lichenin, β-1,3- and β-1,4-glucan
- oat beta-glucan, β-1,3- and β-1,4-glucan
- pleuran, β-1,3- and β-1,6-glucan isolated from Pleurotus ostreatus
- zymosan, β-1,3-glucan
Properties
Properties of glucans include resistance to oral acids/enzyme and water insolubility. Glucans extracted from grains tend to be both soluble and insoluble. [clarification needed]
Structure
Glucans are polysaccharides derived from glucose monomers. The monomers are linked by glycosidic bonds. Four types of glucose-based polysaccharides are possible: 1,6- (starch), 1,4- (cellulose), 1,3- (laminarin), and 1,2-bonded glucans.
thumb
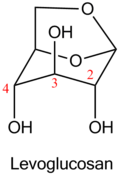
The first representatives of main chain unhydrolysable linear polymers made up of levoglucosan units were synthesized in 1985 by anionic polymerization of 2,3-epoxy derivatives of levoglucosan (1,6;2,3-dianhydro-4-O-alkyl-β-D-mannopyranoses).[5]
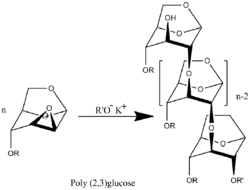
A wide range of unique monomers with different radical R can be synthesized.[6] There were synthesized polymers with R= -CH3,[5] -CH2CHCH2,[7] and -CH2C6H5.[8] Investigation of the polymerization kinetics of those derivatives, molecular weight and molecular-weight distribution showed that the polymerization has the features of a living polymerization system. The process takes place without termination and transfer of the polymer chain with a degree of polymerization equal to the mole ratio of the monomer to the initiator.[9][10] Accordingly, the upper value molecular weight polymer determines only degree of purification system what determine the presence in the system uncontrollable amount of terminators of polymer chains.
Poly(2-3)-D-glucose was synthesized proceeds by transformation of benzyl (R= -CH2C6H5) functionalized polymer.[8]
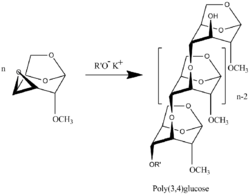
Polymerization of 3,4-epoxy levoglucosan (1,6;3,4-dianhydro-2-O-alkyl-β-D-galactopyranose) results in formation 3,4-bounded levoglucosan polymer.[11]
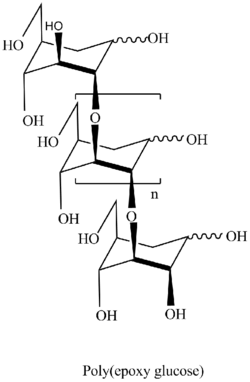
The presence of 1,6-anhydro structure in every unit of polymer chains allows researchers to apply all spectra of well developed methods of carbohydrate chemistry with formation of highly intriguing biological application polymers. The polymers are the only known regular polyethers built up of carbohydrate units in main polymer chain.[12][13]
Biochemical synthesis
Photosynthetic microorganisms, such as cyanobacteria and microalgae, are currently used for their polyglucan production. Since these organisms have high-photosynthetic activity and whole-year cultivation without utilization of arable land. The cultivation is done by modifying the nutrient supply and replacing the growth medium of the cyanobacteria and green microalgae since the control and manipulation of polyglucan metabolism necessitates the elucidation of the polyglucan production mechanism.[14] These activities promote the growth of polyglucans from these organisms.
Several cyanobacteria enzymes could synthesis α-1,2-glucan.[15]
Functions
Glucans serve a diverse set of functions. Within the cell, certain glucans store energy, fortify cellular structure, behave in recognition, and enhance virulence in pathogenic organisms.[16]
Glycogen and starch are notable glucans responsible for storing energy for the cell. Receptor molecules of the immune system, such as the Complement receptor 3, or CR3, and CD5 receptor, recognize and bind to beta-glucans on invading cell surfaces.[17]
Polyglucans are utilized as a carbon source for microbial fermentation. Although polyglucan production has so far been promoted by nutrient limitation, it must be further enhanced to accommodate market demand. The combined strategies of cultivation design and genetic engineering are used for polyglucan productivity for bioethanol production.[14]
Polyglucans are also involved in another sector of the energy industry, acting as biopolymers to increase oil recovery. The polysaccharide is attached to the bacteria cells and then mixed in an alkali solution such as sodium hydroxide to become soluble. After which, it is then pumped into the injection well. The reason it needs to be a fluid is so you can pump the polysaccharides into the reservoir, but then the polysaccharide needs to gel/solidify/precipitate in situ upon addition of another chemical in order to plug up the pore. The biopolymer is then combined and injected with water until it fills up at least 30% of the empty pores. Next, there is an injection of an acid solution or CO
2 forming HCO−
3. This neutralizes the solution and allows for the precipitation of the biopolymer, polyglucans, inside the high-permeability zones. Evidence shows that the application of this polyglucan can reduce the permeability of approximately 80% of the high-permeability zones.[18] Oil companies are able to benefit from the decreased permeability because oil tends to flow in areas with the highest permeability. They can also serve as dietary supplements.
Clinical significance
The immune-modulation action of polyglucans has been known for over 40 years, after experiments showed that they stimulated the activation of macrophages through the activation of the complement system.[3] The detection of the (1,3)-β-D-glucan in blood is also used as a means of identifying invasive or disseminated fungal infections.[19][20] Although, a positive test does not render a diagnosis, and a negative test does not rule out infection. This test can aid in the detection of Aspergillus, Candida, and Pneumocystis jirovecii.[21]
See also
References
- ↑ Glucans at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Glosbe. English Dictionary. https://glosbe.com/en/en/polyglucan. Retrieved 6 February 2011.
- ↑ 3.0 3.1 Longevie. Polyglucan. https://www.longevie.com/en/specialities/5-polyglucan-pot-de-60-capsules-.html.
- ↑ Synytsya A, Novak M. Structural analysis of glucans. Ann Transl Med. 2014 Feb; 2(2):17. doi:10.3978/j.issn.2305-5839.2014.02.07.
- ↑ 5.0 5.1 Berman, E.L., Gorkovenko A.A., Zubov, V.P., and Ponomarenko, V.A., "Regio and Stereospecific Synthesis of Polyglucose with Novel Type Bond" Soviet J.Bioorg. Chem. 11 (1985), 1125-1129
- ↑ Carlson, LJ (November 1965). "Preparation of 2- and 4-Substituted D-Glucose Derivatives from 1,6-Anhydro-β-D- glucopyranose". The Journal of Organic Chemistry 30 (11): 3953–3955. doi:10.1021/jo01022a517.
- ↑ Gorkovenko, A.A., Berman, E.L., and Ponomarenko, V.A. Polymerization of 1, 6;2,3 dianhydro 4 O allyl β D manno¬pyranose Vysocomol. Soed., Ser. B, 1987, 29, 134 137
- ↑ 8.0 8.1 Gorkovenko, A.A., Berman, E.L., and Ponomarenko, V.A. "A New Polymer of Glucose. Poly(2 3) D glucose" Soviet J. Bioorg. Chem., 1987, 13, 218 222
- ↑ Berman, E.L., Gorkovenko, A.A., Rogozhkina, E.D., Izumnikov, A.A., and Ponomarenko, V.A. "Kinetics and Mechanism of Epoxy Ring-Opening Polymerization of 1,6;2,3-Dianhydro-4-O-alkyl-b-D-mannopyranoses" Polymer Sci. USSR, 1988, 413-418
- ↑ Berman E.L. Gorkovenko, A.A., Rogozhkina, E.D., Izyumnikov, A.L., and Ponomarenko, V.A. "Synthesis of Chiral Derivatives of Poly(Ethylene Oxide)" Bull. Acad. Sci. USSR, Div. Chem. Sci., 1988, 705 707
- ↑ Gorkovenko A. A., Berman, E.L., and Ponomarenko, V.A., Poly(3 4) 2 O methyl 1,6 anhydro b D glucopyranose. The First Example of (3 4) linked Polymer Carbohydrates" Soviet J. Bioorg. Chem. 12 (1986), 514-520
- ↑ Berman E.L., Gorkovenko, A.A., and Ponomarenko, V.A. "Structure and Polymerizability of 1,6;2,3 and 1,6;3,4¬ Dianhydrohexapyranoses" Polymer Sci. USSR, 1988, 30, 497¬-502
- ↑ Berman, E.L., "New Glucose Polymers" in "Levoglucosenone and Levoglucosanes: Symposium: 204th National meeting", Zbigniew J. Witczak (editor), American Chemical Society. Division of Carbohydrate Chemistry, 189-214. Publisher: A T L Press, Scientific Publishers ISBN 978-1-882360-13-0 ISBN 1882360133
- ↑ 14.0 14.1 Ho, ShihHsin; Aikawa, Shimpei; Kondo, Akihiko (2015). Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, New Jersey: John Wiley & Sons.
- ↑ Fischer, D., Geyer, A. and Loos E., "Occurrence of glucosylsucrose [alpha-D-glucopyranosyl- (1-->2)-alpha-D-glucopyranosyl-(1-->2)-beta-D-fructofuranoside] and glucosylated homologues in cyanobacteria. Structural properties, cellular contents and possible function as thermoprotectants"FEBS J. 2006 Jan;273(1):137-49.
- ↑ Ruiz-Herrera, José; Ortiz-Castellanos, Lucila (2019-12-01). "Cell wall glucans of fungi. A review" (in en). The Cell Surface 5. doi:10.1016/j.tcsw.2019.100022. ISSN 2468-2330. PMID 32743138.
- ↑ Goodridge, Helen; Wolf, Andrea; Underhill, David (29 June 2009). "β-glucan recognition by the innate immune system". Immunological Reviews 230 (1): 38–50. doi:10.1111/j.1600-065X.2009.00793.x. ISSN 0105-2896. PMID 19594628.
- ↑ Xu, J. Materials for Microcellular Injection Molding, in Microcellular Injection Molding.
- ↑ "Multicenter clinical evaluation of the (1→3)β-D-glucan assay as an aid to diagnosis of fungal infections in humans". Clin Infect Dis 41 (5): 654–659. 2005. doi:10.1086/432470. PMID 16080087.
- ↑ "Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome". Clin Infect Dis 39 (2): 199–205. 2004. doi:10.1086/421944. PMID 15307029.
- ↑ Lahmer, Tobias; da Costa, Clarissa Prazeres; Held, Jürgen; Rasch, Sebastian; Ehmer, Ursula; Schmid, Roland M.; Huber, Wolfgang (2017-04-04). "Usefulness of 1,3 Beta-D-Glucan Detection in non-HIV Immunocompromised Mechanical Ventilated Critically Ill Patients with ARDS and Suspected Pneumocystis jirovecii Pneumonia". Mycopathologia 182 (7–8): 701–708. doi:10.1007/s11046-017-0132-x. ISSN 1573-0832. PMID 28378239.
 |
