Biology:Myosin light-chain kinase
| Myosin Light-Chain kinase, smooth muscle | |
|---|---|
| Identifiers | |
| Symbol | MYLK |
| NCBI gene | 4638 |
| HGNC | 7590 |
| OMIM | 600922 |
| RefSeq | NM_053025 |
| UniProt | Q15746 |
| Other data | |
| EC number | 2.7.11.18 |
| Locus | Chr. 3 qcen-q21 |
| myosin light-chain kinase 2, skeletal muscle | |
|---|---|
 | |
| Identifiers | |
| Symbol | MYLK2 |
| NCBI gene | 85366 |
| HGNC | 16243 |
| OMIM | 606566 |
| RefSeq | NM_033118 |
| UniProt | Q9H1R3 |
| Other data | |
| Locus | Chr. 20 q13.31 |
| myosin light-chain kinase 3, cardiac | |
|---|---|
| Identifiers | |
| Symbol | MYLK3 |
| NCBI gene | 91807 |
| HGNC | 29826 |
| OMIM | 612147 |
| RefSeq | NM_182493 |
| UniProt | Q32MK0 |
| Other data | |
| Locus | Chr. 16 q11.2 |
| Human Myosin Light-Chain Kinase | |
|---|---|
 The Crystal Structure of the Human Myosin Light Chain Kinase Loc340156.[2] | |
| Identifiers | |
| Symbol | MYLK4 |
| NCBI gene | 340156 |
| HGNC | 27972 |
| RefSeq | NM_001012418 |
| UniProt | Q86YV6 |
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II.[3]
General structural features
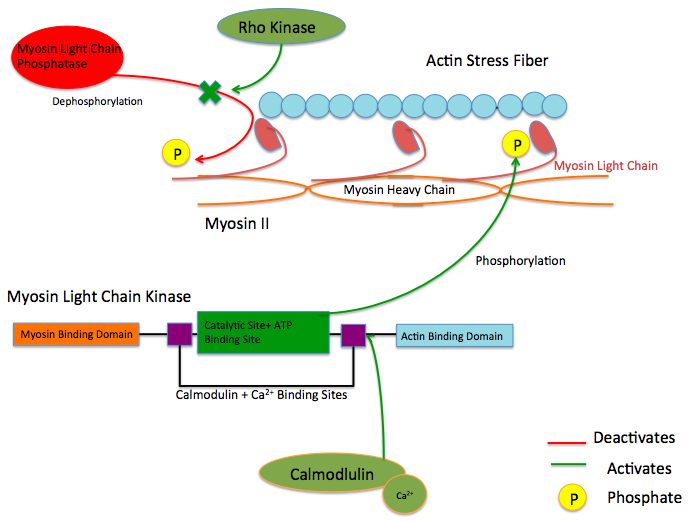
While there are numerous differing domains depending on the cell type, there are several characteristic domains common amongst all MYLK isoforms. MYLK’s contain a catalytic core domain with an ATP binding domain. On either sides of the catalytic core sit calcium ion/calmodulin binding sites. Binding of calcium ion to this domain increases the affinity of MYLK binding to myosin light chain. This myosin binding domain is located at the C-Terminus end of the kinase. On the other side of the kinase at the N-Terminus end, sits the actin-binding domain, which allows MYLK to form interactions with actin filaments, keeping it in place.[4][5]
Isoforms
Four different MYLK isoforms exist:[6]
Function
These enzymes are important in the mechanism of contraction in muscle. Once there is an influx of calcium cations (Ca2+) into the muscle, either from the sarcoplasmic reticulum or from the extracellular space, contraction of smooth muscle fibres may begin. First, the calcium will bind to calmodulin.[7] After the influx of calcium ions and the binding to calmodulin, pp60 SRC (a protein kinase) causes a conformational change in MYLK, activating it and resulting in an increase in phosphorylation of myosin light chain at serine residue 19. The phosphorylation of MLC will enable the myosin crossbridge to bind to the actin filament and allow contraction to begin (through the crossbridge cycle). Since smooth muscle does not contain a troponin complex, as striated muscle does, this mechanism is the main pathway for regulating smooth muscle contraction. Reducing intracellular calcium concentration inactivates MLCK but does not stop smooth muscle contraction since the myosin light chain has been physically modified through phosphorylation(and not via ATPase activity). To stop smooth muscle contraction this change needs to be reversed. Dephosphorylation of the myosin light chain (and subsequent termination of muscle contraction) occurs through activity of a second enzyme known as myosin light-chain phosphatase (MLCP).[8]
Upstream Regulators
Protein kinase C and ROC Kinase are involved in regulating Calcium ion intake; these Calcium ions, in turn stimulate a MYLK, forcing a contraction.[9] Rho kinase also modulates the activity of MYLK by downregulating the activity of MYLK's counterpart protein: Myosin Light Chain Phosphatase (MYLP).[10] In addition to downregulation of MYLK, ROCK indirectly strengthens actin/myosin contraction through inhibiting Cofilin, a protein which depolymerizes actin stress fibers.[11] Similar to ROCK, Protein Kinase C regulates MYLK via the CPI-17 protein, which downregulates MYLP.[12]
Mutations and resulting diseases
Some pulmonary disorders have been found to arise due to an inability of MYLK to function properly in lung cells. Over-activity in MYLK creates an imbalance in mechanical forces between adjacent endothelial and lung tissue cells. An imbalance may result in acute respiratory distress syndrome, in which fluid is able to pass into the alveoli.[13] Within the cells, MYLK provides an inward pulling force, phosphorylating myosin light chain causing a contraction of the myosin/actin stress fiber complex. Conversely, cell-cell adhesion via tight and adherens junctions, along with anchoring to extra cellular matrix (ECM) via integrins and focal adhesion proteins results in an outward pulling force. Myosin light chain pulls the actin stress fiber attached to the cadherin, resisting the force of the adjacent cell's cadherin. However, when the inward pulling force of the actin stress fiber becomes greater than the outward pulling force of the cell adhesion molecules due to an overactive MYLK, tissues can become slightly pulled apart and leaky, leading to passage of fluid into the lungs.[14]
Another source of smooth muscle disorders like ischemia–reperfusion, hypertension, and coronary artery disease arise when mutations to protein kinase C (PKC) result in excessive inhibition of MYLP, which counteracts the activity of MYLK by dephosphorylating myosin light chain. Because myosin light chain has no inherent phosphate cleaving property over active PKC prevents the dephosphorylation of myosin light protein leaving it in the activated conformation, causing an increase in smooth muscle contraction.[12]
See also
References
- ↑ Radu, L.; Assairi, L.; Blouquit, Y.; Durand, D.; Miron, S.; Charbonnier, J.B.; Craescu, C.T. (2011). "RCSB Protein Data Bank - Structure Summary for 3KF9 - Crystal structure of the SdCen/skMLCK complex". Worldwide Protein Data Bank. doi:10.2210/pdb3kf9/pdb. https://www.rcsb.org/pdb/explore/explore.do?structureId=3KF9.
- ↑ Muniz, J.R.C.; Mahajan, P.; Rellos, P.; Fedorov, O.; Shrestha, B.; Wang, J.; Elkins, J.M.; Daga, N. et al. (2010). "RCSB Protein Data Bank - Structure Summary for 2X4F - The Crystal Structure of the Human Myosin Light Chain Kinase Loc340156.". Worldwide Protein Data Bank. doi:10.2210/pdb2x4f/pdb. https://www.rcsb.org/pdb/explore/explore.do?structureId=2X4F.
- ↑ "Myosin light chain kinase as a multifunctional regulatory protein of smooth muscle contraction". IUBMB Life 51 (6): 337–44. June 2001. doi:10.1080/152165401753366087. PMID 11758800.
- ↑ "Myosin Light Chain Kinase MYLK1: Anatomy, Interactions, Functions, and Regulation". Biochemistry. Biokhimiia 81 (13): 1676–1697. December 2016. doi:10.1134/S000629791613006X. PMID 28260490.
- ↑ "Myosin Light Chain Kinase: Functional Domains and Structural Motifs". Acta Physiologica 164 (4): 471–482. December 1998. doi:10.1111/j.1365-201X.1998.tb10699.x. PMID 9887970.
- ↑ "The protein kinase complement of the human genome". Science 298 (5600): 1912–34. December 2002. doi:10.1126/science.1075762. PMID 12471243. Bibcode: 2002Sci...298.1912M.
- ↑ Robinson, A; Colbran, R (2013). "Calcium/Calmodulin-Dependent Protein Kinases". in Lennarz, William; Lane, Daniel. Encyclopedia of Biological Chemistry (2nd ed.). Elsevier inc.. pp. 304–309. ISBN 978-0-12-378631-9.
- ↑ Feher, Joseph (2017). "Smooth Muscle". Quantitative Human Physiology (2nd ed.). Elsevier inc.. pp. 351–361. ISBN 978-0-12-800883-6.
- ↑ "Calcium sensitization mechanisms in detrusor smooth muscles". Journal of Basic and Clinical Physiology and Pharmacology 29 (3): 227–235. January 2018. doi:10.1515/jbcpp-2017-0071. PMID 29306925.
- ↑ "Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity". Cytoskeleton 67 (9): 545–54. September 2010. doi:10.1002/cm.20472. PMID 20803696.
- ↑ "Cytoskeletal regulation of pulmonary vascular permeability". Journal of Applied Physiology 91 (4): 1487–500. October 2001. doi:10.1152/jappl.2001.91.4.1487. PMID 11568129.
- ↑ 12.0 12.1 "Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders". Vascular Pharmacology - Smooth Muscle. Advances in Pharmacology. 78. 2017. pp. 203–301. doi:10.1016/bs.apha.2016.06.002. ISBN 978-0-12-811485-8.
- ↑ "Epigenetic contribution of the myosin light chain kinase gene to the risk for acute respiratory distress syndrome". Translational Research 180: 12–21. February 2017. doi:10.1016/j.trsl.2016.07.020. PMID 27543902.
- ↑ "Myosin Light Chain Kinase: Pulling the Strings of Epithelial Tight Junction Function". Annals of the New York Academy of Sciences 1258 (1): 34–42. July 2012. doi:10.1111/j.1749-6632.2012.06526.x. PMID 22731713. Bibcode: 2012NYASA1258...34C.
Further reading
- "A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability". The Journal of Biological Chemistry 279 (53): 55506–13. December 2004. doi:10.1074/jbc.M408822200. PMID 15507455.
- "Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression". The American Journal of Pathology 166 (2): 409–19. February 2005. doi:10.1016/S0002-9440(10)62264-X. PMID 15681825.
- "Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure". Gastroenterology 128 (4): 987–1001. April 2005. doi:10.1053/j.gastro.2005.01.004. PMID 15825080.
- "Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells". The Journal of Physiology 570 (Pt 2): 219–35. January 2006. doi:10.1113/jphysiol.2005.097998. PMID 16284075.
- "Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells". American Journal of Physiology. Cell Physiology 290 (4): 1031–40. April 2006. doi:10.1152/ajpcell.00602.2004. PMID 16306123.
- "Myosin light chain kinase plays a role in the regulation of epithelial cell survival". Journal of Cell Science 119 (Pt 11): 2269–81. June 2006. doi:10.1242/jcs.02926. PMID 16723733. https://repository.cshl.edu/22776/1/Helfman%20J%20Cell%20Science%202006.pdf.
- "A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart". The Journal of Clinical Investigation 117 (10): 2812–24. October 2007. doi:10.1172/JCI30804. PMID 17885681.
- "Biochemistry of Smooth Muscle Myosin Light Chain Kinase". Archives of Biochemistry and Biophysics 510 (2): 135–146. 15 June 2011. doi:10.1016/j.abb.2011.04.018. ISSN 0003-9861. PMID 21565153.
External links
- MYLK+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
- Myosin-Light-Chain+Kinase at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |

