Biology:Ochrophyte
| Ochrophytes | |
|---|---|

| |
| Dense kelp forest with understory at Partridge Point near Dave's Caves, Cape Peninsula | |
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | SAR |
| Clade: | Stramenopiles |
| Phylum: | Gyrista |
| Subphylum: | Ochrophytina Cavalier-Smith, 1986 |
| Classes[3] | |
| |
| Diversity | |
| >20,000 species | |
| Synonyms | |
| |
Ochrophytes (also known as heterokontophytes and stramenochromes) are the photosynthetic stramenopiles, a group of eukaryotes characterized by the presence of two unequal flagella, one of which has tripartite hairs called mastigonemes. In particular, ochrophytes are characterized by their plastids enclosed by four membranes, with thylakoids organized in piles of three, and the presence of chlorophylls a, c, and additional pigments such as β-carotene and xanthophylls. Ochrophytes are one of the most diverse lineages of eukaryotes, containing ecologically important algae such as brown algae and diatoms. They are classified either as phylum Ochrophyta or subphylum Ochrophytina within phylum Gyrista. Their plastid is of red algal origin.
Cell biology
The ochrophytes are all photosynthetic stramenopiles. As such, their cells frequently display an anterior flagellum with straw-like tripartite hairs called mastigonemes, and a posterior smooth flagellum, a characteristic of the Stramenopila.[6][7] Due to comprising the entirety of stramenopile algae, they are also known as heterokontophytes or stramenochromes. Ochrophytes accumulate chrysolaminarin as storage product,[7] within cytoplasmic vesicles.[6] Cytoplasmic lipid droplets are also common.[7] They uniformly have tubular mitochondrial cristae.[7]
Plastids and membranes
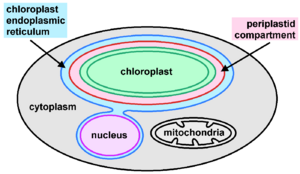
Ochrophytes may possess one or more photosynthetic plastids (chloroplasts) per cell.[8] Some groups contain species with leucoplasts, choroplasts that have lost photosynthetic capacity and pigments but presumably continue to play a role in the synthesis of aminoacids, lipids and heme groups.[7] They have a distinct plastid ultrastructure in comparison to other algal groups.[8] The plastid originates from an event of secondary endosymbiosis between a red algal endosymbiont and a phagotrophic stramenopile. As such, they are typically surrounded by four membranes: two inner membranes, corresponding to the primary plastid membranes; a third membrane, corresponding to the plasma membrane of the red alga; and an outermost layer, corresponding to the phagosome membrane.[9] The two outer layers are continguous with the endoplasmic reticulum (ER), together composing the chloroplast endoplasmic reticulum (CER),[8] also known as the periplastidial endoplasmic reticulum (PER), which is often connected to the nuclear envelope. The tripartite flagellar hairs, characteristic of stramenopiles, are produced within either the PER or the nuclear envelope.[7]
The periplastid compartment (PPC), between the second and third layers, is a separate region that in other algal groups (i.e. cryptomonads and chlorarachniophytes) contains a nucleomorph, the vestigial nucleus of the secondary endosymbiont; however, no nucleomorphs are known within the ochrophytes. Instead, other structures have been observed within the PPC, similarly to those seen in haptophytes and chromerid algae:[8] "blob-like structures" where PPC proteins are localized, and a vesicular network.[9] Within the CER, there is a prominent region of tight direct contacts between the periplastid membrane and the inner nuclear envelope, where lipid transfers might occur, and perhaps exchange of other molecules.[9]
Commonly, within the plastid stroma, three stacked thylakoids differentiate into the "girdle lamella", which runs around the periphery of the plastid, beneath the innermost membrane.[8] The remaining thylakoids are arranged in stacks of three.[7] In synchromophytes and aurearenophytes, a consortium of several plastids, each surrounded by two or three inner membranes respectively, is enveloped by a shared outer membrane.[8]
Pigmentation
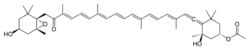
Ochrophyte chloroplasts contain chlorophylls a and c as photosynthetic pigments, in addition to fucoxanthin.[6] Chlorophyll a binds to thylakoids, while the c pigment is present in the stroma.[7] The most frequent accessory pigment in ochrophytes is the yellow β-carotene. The golden-brown or brown pigmentation in diatoms, brown algae, golden algae and others is conferred by the xanthophyll fucoxanthin. In the yellow-green or yellow-brown raphidophyceans, eustigmatophyceans and xanthophyceans, vaucheriaxanthin is dominant instead. These pigment combinations extend their photosynthetic ability beyond chlorophyll a alone. Additionally, xanthophylls protect the photosystems from high intensity light.[7]
Ecology
Representatives of ochrophytes can be found in marine water, freshwater and soils. Some classes are more common in marine habitats, while others are more frequent in freshwater or soil.[7] Among the ochrophyte lineages are the diatoms, the most abundant photosynthetic eukaryotes worldwide in marine habitats; multicellular seaweeds, such as brown algae and golden algae; and an array of microscopic single-celled lineages that are also abundant, according to environmental sequencing.[8]
Harmful algae
Two main lineages of photosynthetic stramenopiles include many toxic species. Within the class Raphidophyceae, strains of Heterosigma and Chattonella at high concentrations are responsible for fish mortality, although the nature and action of their toxins is not resolved. Freshwater Gonyostomum species are capable of mucilage secretion at high amounts detrimental to fish gills. Within the diatoms (Bacillariophyta), harmful effects can be due to physical damage or to toxin production. Centric diatoms like Chaetoceros live as colonial chains of cells with long spines (setae) that can clog fish gills, causing their death. Among diatoms, the only toxin producers have been found among pennate diatoms, almost entirely within the genus Pseudonitzschia. More than a dozen species of Pseudonitzschia are capable of producing a neurotoxin, domoic acid, the cause of amnesiac shellfish poisoning.[10]
Evolution
External
The ochrophytes constitute a highly diverse clade within Stramenopila, a eukaryotic supergroup that also includes several heterotrophic lineages of protists such as oomycetes, hyphochytrids, labyrinthuleans, opalines and bicosoecids.[11][3][12] Stramenopiles, also known as heterokonts, are characterized by the presence of two flagella, one of which has hollow tripartite tubular hairs called mastigonemes, but these are secondarily lost in some groups.[3]
This lineage of stramenopiles originated from an event of secondary endosymbiosis where a red alga was transformed into the chloroplast of the common ancestor of ochrophytes.[3][13][14] The total group of ochrophytes is estimated to have evolved between 874 and 543 million years ago (Ma) through molecular clock inference. However, the earliest fossil remains, assigned to the billion-year-old golden alga Palaeovaucheria,[1] suggest that ochrophytes had appeared by 1000 Ma. Other early putative representatives of photosynthetic stramenopiles are Jacutianema (750 Ma), Germinosphaera (750–700 Ma) and the brown alga Miaohephyton (600–550 Ma). Scales similar to modern chrysophyte scales, and valves resembling the modern centric diatom valves, have been found in 800–700 million-years-old sediments.[15]
Internal
| Ochrophyte phylogeny | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Evolutionary relationships between all ochrophyte classes based on the latest phylogenetic analyses,[17][14][16][2] and the approximate number of species in each class.[3] |
Relationships among many classes of ochrophytes remain unresolved, but three main clades (called SI, SII and SIII) are supported in most phylogenetic analyses. The SI lineage, containing the diverse and multicellular class Phaeophyceae, or brown algae, experienced an evolutionary radiation during the late Paleozoic (around 310 million years ago). The class Schizocladiophyceae is the sister lineage to brown algae, followed by a clade of closely related classes Xanthophyceae, Phaeosacciophyceae[16] and Chrysoparadoxophyceae.[18] This is in turn the sister lineage to a clade containing Aurearenophyceae and Phaeothamniophyceae,[3] which are sometimes treated as one class Aurophyceae.[14] The Raphidophyceae are the most basal within the SI. The SII lineage contains the golden algae or Chrysophyceae, as well as smaller classes Synurophyceae, Eustigmatophyceae, Pinguiophyceae and Picophagea (also known as Synchromophyceae). Both clades, SI and SII, compose the Chrysista lineage. The remaining classes are grouped within the sister lineage Diatomista, equivalent to the SIII lineage; these are the diatoms or Bacillariophyceae, Bolidophyceae, Dictyochophyceae (including the silicoflagellates) and Pelagophyceae.[3] A new class of algae, Olisthodiscophyceae, was described in 2021 and recovered as part of the SII lineage.[2]
One group of heterotrophic heliozoan protists, Actinophryida,[19] is included in some classifications as the sister lineage to the raphidophytes, and both groups are treated as one class Raphidomonadea on the basis of 18S rDNA phylogenetic analyses.[20] However, a recent phylogenomic study places one actinophryid, Actinophrys sol, as the probable sister group to ochrophytes.[21]
Classification
Hierarchical
In hierarchical classifications, where taxonomic ranks (kingdom, phylum, class, order...) are utilized, the ochrophytes are commonly regarded as an entire phylum (or division in botanical nomenclature) by the name of Ochrophyta, within the Stramenopila or Heterokonta.[11] The phylum was first described by protozoologist Thomas Cavalier-Smith in 1986.[22] It remained as a phylum-level taxon until 2018, when the same author lowered it to subphylum level and modified the name to Ochrophytina to match the -phytina suffix in botanical nomenclature, which corresponds to subdivisions. The phylum to which ochrophytes belong in his classification system is Gyrista, a clade that also contains some heterotrophic stramenopiles, namely the Pseudofungi and the Bigyromonada.[14] Gyrista and Bigyra compose the two main branches of stramenopiles, which are regarded as the superphylum Heterokonta within the kingdom Chromista. However, this classification system is in disuse, due to the non-monophyletic nature of the kingdom.[12]
Cladistic
As opposed to the hierarchical classification, the cladistic classification only recognizes clades as valid groups, rejecting the use of paraphyletic or polyphyletic groups. This method of classification is preferred among protistologists. The latest revision of the International Society of Protistologists, in 2019, recognizes Ochrophyta as a valid taxon within the higher Stramenopiles group, within the SAR supergroup.[12]
Below is the present classification of ochrophytes according to the most recent revision of 2019,[12] with the inclusion of three new classes of algae described in posterior years.[18][16][2] The subdivision of ochrophytes between Chrysista and Diatomista is fully accepted by the scientific community and backed up by phylogenetic analyses.[12] According to this revision, the diatoms (Diatomeae) do not form a single class Bacillariophyceae. Instead, they are divided into numerous classes of new description, to reflect the phylogenetic advances over the previous decade.[12]
- Chrysista Cavalier-Smith 1986
- Aurearenophyceae Kai et al. 2008[23]
- Chrysoparadoxophyceae Wetherbee et al. 2019[18]
- Chrysophyceae Pascher 1914
- Eustigmatophyceae Hibberd 1981
- Olisthodiscophyceae Barcytė, Eikrem & M. Eliáš, 2021[2]
- Phaeophyceae Hansgirg 1886
- Phaeosacciophyceae R.A.Andersen, L.Graf & H.S.Yoon 2020[16]
- Phaeothamniophyceae Andersen & Bailey in Bailey et al. 1998[24]
- Raphidophyceae Cahdefaud 1950, emend. Silva 1980
- Schizocladiophyceae Kawai et al. 2003[25]
- Xanthophyceae Allorge 1930 emend. Fritsch 1935 (=Heterokontae Luther 1899; Heteromonadea Leedale 1983; Xanthophyta Hibberd 1990; Tribophyceae[26])
- Diatomista Derelle et al. 2016, emend. Cavalier-Smith 2017
- Dictyochophyceae Silva 1980
- Pelagophyceae Andersen & Saunders 1993
- Pinguiophyceae Kawachi et al. 2003
- Bolidophyceae Guillou et al. 1999
- Diatomeae Dumortier 1821 (=Bacillariophyta Haeckel 1878)
- Leptocylindrophytina D.G. Mann in Adl et al. 2019
- Leptocylindrophyceae D.G. Mann in Adl et al. 2019
- Corethrophyceae D.G. Mann in Adl et al. 2019
- Ellerbeckiophytina D.G. Mann in Adl et al. 2019
- Probosciophytina D.G. Mann in Adl et al. 2019
- Melosirophytina Medlin & Kaczmarska 2004, emend. Adl et al. 2019
- Coscinodiscophytina D.G. Mann in Adl et al. 2019
- Rhizosoleniophytina D.G. Mann in Adl et al. 2019
- Arachnoidiscophytina D.G. Mann in Adl et al. 2019
- Bacillariophytina Medlin & Kaczmarska 2004, emend. Adl et al. 2019
- Mediophyceae Jouse & Proshkina-Lavrenko in Medlin & Kaczmarska 2004
- Biddulphiophyceae D.G. Mann in Adl et al. 2019
- Bacillariophyceae Haeckel 1878, emend. Adl et al. 2019
- Leptocylindrophytina D.G. Mann in Adl et al. 2019
Knowledge
Multicellular seaweeds, in the class Phaeophyceae, were described in early Chinese (around 3000 BC), Greek (300 BC, such as Theophrastus) and Japanese (ca. 500 AD) writings. Their knowledge likely predates recorded history, being used as food, dyes and for medicinal purposes. The first formal description of a stramenopile alga was that of the genus Fucus, by Linnaeus in his 1753 work Species Plantarum. Shortly after, unicellular chrysophytes were described by Otto Friedrich Müller. During this first era of scientific discovery, brown algae were described as plants, while microscopic algae were treated as animals under the name of infusoria.[27]
During the 20th century, evolutionary and phylogenetic discussions began including heterokont algae. Transmission electron microscopy and molecular phylogenetic analysis led to the description of many new groups and several classes well into the 21st century. In 2002 began the sequencing of the first ochrophyte genome, belonging to Thalassiosira pseudonana.[27]
References
- ↑ Jump up to: 1.0 1.1 "A Vaucheriacean Alga from the Middle Neoproterozoic of Spitsbergen: Implications for the Evolution of Proterozoic Eukaryotes and the Cambrian Explosion". Paleobiology 30 (2): 231–252. 2004. http://www.jstor.org/stable/4096845.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 Dovilė Barcytė; Wenche Eikrem; Anette Engesmo; Sergio Seoane; Jens Wohlmann; Aleš Horák; Tatiana Yurchenko; Marek Eliáš (2 March 2021). "Olisthodiscus represents a new class of Ochrophyta". Journal of Phycology 57 (4): 1094–1118. doi:10.1111/jpy.13155. PMID 33655496.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Phylogeny and Evolution of the Brown Algae". Critical Reviews in Plant Sciences 39 (4): 281–321. 2020. doi:10.1080/07352689.2020.1787679.
- ↑ Cavalier-Smith, T. (1986). The kingdom Chromista, origin and systematics. In: Round, F.E. and Chapman, D.J. (eds.). Progress in Phycological Research. 4: 309–347.
- ↑ Jump up to: 5.0 5.1 Reviers, B. de. (2006). Biologia e Filogenia das Algas. Editora Artmed, Porto Alegre, p. 157.
- ↑ Jump up to: 6.0 6.1 6.2 Phycology (5th ed.). Cambridge University Press. 2018. doi:10.1017/9781316407219. ISBN 978-1-107-55565-5.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9 "Photosynthetic Stramenopiles I: Introduction to Photosynthetic Stramenopiles". Algae (4th ed.). LJLM Press. 2022. pp. 12-2–12-4. ISBN 978-0-9863935-4-9.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 8.6 "Chapter Three - Secondary Plastids of Stramenopiles". Advances in Botanical Research 84: 57–103. 2017. doi:10.1016/bs.abr.2017.06.003.
- ↑ Jump up to: 9.0 9.1 9.2 "Ultrastructure of the Periplastidial Compartment of the Diatom Phaeodactylum tricornutum". Protist 167 (1): 254–267. 2016. doi:10.1016/j.protis.2016.04.001.
- ↑ "Biodiversity of Harmful Marine Algae". Encyclopedia of Biodiversity (Second ed.). Academic Press. 2013. pp. 470–484. doi:10.1016/B978-0-12-384719-5.00404-4. ISBN 9780123847201.
- ↑ Jump up to: 11.0 11.1 Ingvild Riisberga; Russell J. S. Orr; Ragnhild Kluge; Kamran Shalchian-Tabrizi; Holly A. Bowers; Vishwanath Patil; Bente Edvardsen; Kjetill S. Jakobsen (2009). "Seven gene phylogeny of heterokonts". Protist 160 (2): 191–204. doi:10.1016/j.protis.2008.11.004. PMID 19213601.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 "Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes". Journal of Eukaryotic Microbiology 66 (1): 4–119. 2019. doi:10.1111/jeu.12691. PMID 30257078.
- ↑ "Updating algal evolutionary relationships through plastid genome sequencing: did alveolate plastids emerge through endosymbiosis of an ochrophyte?". Sci Rep 5: 10134. 2015. doi:10.1038/srep10134. PMID 26017773. Bibcode: 2015NatSR...510134S.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 Cavalier-Smith, Thomas (2018). "Kingdom Chromista and its eight phyla: a new synthesis emphasising periplastid protein targeting, cytoskeletal and periplastid evolution, and ancient divergences". Protoplasma 255 (1): 297–357. doi:10.1007/s00709-017-1147-3. PMID 28875267.
- ↑ "A Molecular Genetic Timescale for the Diversification of Autotrophic Stramenopiles (Ochrophyta): Substantive Underestimation of Putative Fossil Ages". PLoS ONE 5 (9): e12759. 2010. doi:10.1371/journal.pone.0012759.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 "Multigene Phylogeny, Morphological Observation and Re-examination of the Literature Lead to the Description of the Phaeosacciophyceae Classis Nova and Four New Species of the Heterokontophyta SI Clade". Protist 171 (6): 125781. December 2020. doi:10.1016/j.protis.2020.125781. PMID 33278705.
- ↑ "A Phylogenomic Framework to Study the Diversity and Evolution of Stramenopiles (=Heterokonts)". Mol Biol Evol 33 (11): 2890–2898. November 2016. doi:10.1093/molbev/msw168. PMID 27512113.
- ↑ Jump up to: 18.0 18.1 18.2 "The golden paradox - a new heterokont lineage with chloroplasts surrounded by two membranes". J Phycol 55 (2): 257–278. April 2019. doi:10.1111/jpy.12822. PMID 30536815.
- ↑ Mikrjukov, Kirill A.; Patterson, David J. (2001). "Taxonomy and phylogeny of Heliozoa. III. Actinophryids". Acta Protozoologica 40: 3–25. http://www.bio-nica.info/biblioteca/Mikrjukov2001.pdf.
- ↑ Cavalier-Smith, Thomas; Scoble, Josephine Margaret (2012). "Phylogeny of Heterokonta: Incisomonas marina, a uniciliate gliding opalozoan related to Solenicola (Nanomonadea), and evidence that Actinophryida evolved from raphidophytes". European Journal of Protistology 49 (3): 328–353. doi:10.1016/j.ejop.2012.09.002. PMID 23219323.
- ↑ Azuma, Tomonori; Pánek, Tomáš; Tice, Alexander K.; Kayama, Motoki; Kobayashi, Mayumi; Miyashita, Hideaki; Suzaki, Toshinobu; Yabuki, Akinori et al. (10 April 2022). "An Enigmatic Stramenopile Sheds Light on Early Evolution in Ochrophyta Plastid Organellogenesis". Molecular Biology and Evolution 39 (4). doi:10.1093/molbev/msac065. PMID 35348760.
- ↑ Thomas Cavalier-Smith; Ema E.-Y. Chao (2006). "Phylogeny and megasystematics of phagotrophic heterokonts (kingdom Chromista)". Journal of Molecular Evolution 62 (4): 388–420. doi:10.1007/s00239-004-0353-8. PMID 16557340. Bibcode: 2006JMolE..62..388C.
- ↑ "Aurearenophyceae classis nova, a New Class of Heterokontophyta Based on a New Marine Unicellular Alga Aurearena cruciata gen. et sp. nov. Inhabiting Sandy Beaches". Protist 159 (3): 435–457. 2008. doi:10.1016/j.protis.2007.12.003. ISSN 1434-4610. PMID 18358776. https://www.sciencedirect.com/science/article/pii/S1434461008000035.
- ↑ "Phaeothamniophyceae Classis Nova: A New Lineage of Chromophytes Based upon Photosynthetic Pigments, rbcL Sequence Analysis and Ultrastructure". Protist 149 (3): 245–63. September 1998. doi:10.1016/S1434-4610(98)70032-X. PMID 23194637.
- ↑ Kawai, Hiroshi; Maeba, Shunsuke; Sasaki, Hideaki; Okuda, Kazuo; Henry, Eric C. (2003). "Schizocladia ischiensis: A New Filamentous Marine Chromophyte Belonging to a New Class, Schizocladiophyceae". Protist 154 (2): 211–228. doi:10.1078/143446103322166518. ISSN 1434-4610. PMID 13677449. https://www.sciencedirect.com/science/article/pii/S1434461004701364.
- ↑ "Notes on the taxonomy and nomenclature of the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae)". Botanical Journal of the Linnean Society 82 (2): 93–119. February 1981. doi:10.1111/j.1095-8339.1981.tb00954.x. https://academic.oup.com/botlinnean/article-abstract/82/2/93/2725653?redirectedFrom=fulltext.
- ↑ Jump up to: 27.0 27.1 "Biology and systematics of heterokont and haptophyte algae". American Journal of Botany 91 (10): 1508–1522. doi:10.3732/ajb.91.10.1508.
External links
| Wikimedia Commons has media related to Ochrophyta. |
Wikidata ☰ {{{from}}} entry
 |