Biology:Plant lipid transfer proteins
| Plant lipid transfer protein / bifunctional inhibitor / seed storage protein, 4-helical domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
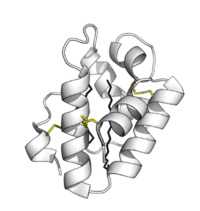
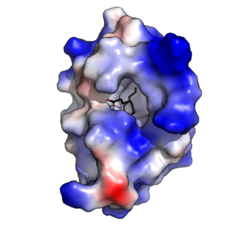 Oryza sativa Lipid Transfer Protein 1 bound to palmitic acid (black). Positive charge in blue; negative charge in red (PDB: 1UVB). | |||||||||||
| Identifiers | |||||||||||
| Symbol | LTP/seed_store/tryp_amyl_inhib | ||||||||||
| Pfam | PF00234 | ||||||||||
| Pfam clan | CL0482 | ||||||||||
| InterPro | IPR016140 | ||||||||||
| SMART | SM00499 | ||||||||||
| CATH | 1UVB | ||||||||||
| SCOP2 | 1UVB / SCOPe / SUPFAM | ||||||||||
| CDD | cd00010 | ||||||||||
| |||||||||||
| Also Pfam PF13016, PF14368; see the Pfam clan relationships. | |||||||||||
Plant lipid transfer proteins, also known as plant LTPs or PLTPs, are a group of highly-conserved proteins of about 7-9kDa found in higher plant tissues.[1][2] As its name implies, lipid transfer proteins facilitate the shuttling of phospholipids and other fatty acid groups between cell membranes.[3] LTPs are divided into two structurally related subfamilies according to their molecular masses: LTP1s (9 kDa) and LTP2s (7 kDa).[4] Various LTPs bind a wide range of ligands, including fatty acids with a C10–C18 chain length, acyl derivatives of coenzyme A, phospho- and galactolipids, prostaglandin B2, sterols, molecules of organic solvents, and some drugs.[2]
The LTP domain is also found in seed storage proteins (including 2S albumin, gliadin, and glutelin) and bifunctional trypsin/alpha-amylase inhibitors.[5][6][7][8] These proteins share the same superhelical, disulfide-stabilised four-helix bundle containing an internal cavity.
There is no sequence similarity between animal and plant LTPs. In animals, cholesteryl ester transfer protein, also called plasma lipid transfer protein, is a plasma protein that facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins.
Function
Ordinarily, most lipids do not spontaneously exit membranes because their hydrophobicity makes them poorly soluble in water. LTPs facilitate the movement of lipids between membranes by binding, and solubilising them. LTPs typically have broad substrate specificity and so can interact with a variety of different lipids.[9]
LTPs are known to be pathogenesis-related proteins, i.e. proteins produced for pathogen defense by plants. Some LTPs are known to be antibacterial, antifungal, antiviral, and/or in vitro antiproliferative.[2] The enzyme inhibitor members are thought to regulate the development and germination of seeds as well as protect against insects and herbivores.[2]
LTPs in plants may be involved in:
- cutin biosynthesis
- surface wax formation
- mitochondrial growth
- adaptation to environmental changes[10]
- lipid metabolism
- fertilization of flowering plants
- adaptation of plants under stress conditions
- activation and regulation of signaling cascades
- apoptosis
- symbiosis
- fruit ripening[2]
Structure
Plant lipid transfer proteins consist of 4 alpha-helices in a right-handed superhelix with a folded leaf topology. The structure is stabilised by disulfide bridges linking the helices to each other.
The structure forms an internal hydrophobic cavity in which 1-2 lipids can be bound. The outer surface of the protein is hydrophilic, allowing the complex to be soluble. The use of hydrophobic interactions, with very few charged interactions, allows the protein to have broad specificity for a range of lipids.[9]
Role in human health
PLTPs are pan-allergens,[11][12] and may be directly responsible for cases of food allergy. Pru p 3, the major allergen from peach, is a 9-kDa allergen belonging to the family of lipid-transfer proteins.[13] Allergic properties are closely linked with high thermal stability and resistance to gastrointestinal proteolysis of the proteins.[14] They are class 1 (gastrointestinal) food allergens that cause a more systemic response than class 2 (respiratory) allergens.[4]
Plant LTPs are considered antioxidants in a small subset of researches.[15] Whether this has value for human health is unknown.
Commercial importance
Lipid transfer protein 1 (from barley) is responsible, when denatured by the mashing process, for the bulk of foam which forms on top of beer.[16]
References
- ↑ "Lipid transfer protein: a pan-allergen in plant-derived foods that is highly resistant to pepsin digestion". International Archives of Allergy and Immunology 124 (1–3): 67–9. 2001. doi:10.1159/000053671. PMID 11306929.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Lipid Transfer Proteins As Components of the Plant Innate Immune System: Structure, Functions, and Applications". Acta Naturae 8 (2): 47–61. 2016. doi:10.32607/20758251-2016-8-2-47-61. PMID 27437139.
- ↑ "Lipid-Transfer Protein in Plants". Annual Review of Plant Physiology and Plant Molecular Biology 47: 627–654. June 1996. doi:10.1146/annurev.arplant.47.1.627. PMID 15012303.
- ↑ 4.0 4.1 "Plant Pathogenesis-Related Proteins PR-10 and PR-14 as Components of Innate Immunity System and Ubiquitous Allergens". Current Medicinal Chemistry 24 (17): 1772–1787. 2017-07-04. doi:10.2174/0929867323666161026154111. PMID 27784212.
- ↑ "Characterization and structural analyses of nonspecific lipid transfer protein 1 from mung bean". Biochemistry 44 (15): 5703–12. April 2005. doi:10.1021/bi047608v. PMID 15823028.
- ↑ "Solution structure of RicC3, a 2S albumin storage protein from Ricinus communis". Biochemistry 42 (47): 13839–47. December 2003. doi:10.1021/bi0352217. PMID 14636051.
- ↑ "Tertiary and quaternary structures of 0.19 alpha-amylase inhibitor from wheat kernel determined by X-ray analysis at 2.06 A resolution". Biochemistry 36 (44): 13503–11. November 1997. doi:10.1021/bi971307m. PMID 9354618.
- ↑ "Structure of the bifunctional inhibitor of trypsin and alpha-amylase from ragi seeds at 2.2 A resolution". Acta Crystallographica D 56 (Pt 3): 287–93. March 2000. doi:10.1107/s0907444999016601. PMID 10713515.
- ↑ 9.0 9.1 "Lipid binding in rice nonspecific lipid transfer protein-1 complexes from Oryza sativa". Protein Science 13 (9): 2304–15. September 2004. doi:10.1110/ps.04799704. PMID 15295114.
- ↑ Kader, Jean-Claude (February 1997). "Lipid-transfer proteins: A puzzling family of plant proteins". Trends in Plant Science 2 (2): 66–70. doi:10.1016/S1360-1385(97)82565-4.
- ↑ Morris, Adrian. "Food Allergy in Detail". Surrey Allergy Clinic. https://www.allergy-clinic.co.uk/allergies/food-allergy/food-allergy-guide/.
- ↑ InterPro: IPR000528
- ↑ Besler, Matthias; Herranz, Javier Cuesta; Fernández-Rivas, Montserrat (2000). "Peach allergy". Internet Symposium on Food Allergens 2 (4): 185–201. http://www.food-allergens.de/symposium-2-4/peach/peach-allergens.htm.
- ↑ "A novel lipid transfer protein from the pea Pisum sativum: isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic properties". BMC Plant Biology 16: 107. April 2016. doi:10.1186/s12870-016-0792-6. PMID 27137920.
- ↑ "Antioxidants in human health and disease". Annual Review of Nutrition 16: 33–50. 1996. doi:10.1146/annurev.nu.16.070196.000341. PMID 8839918.
- ↑ "Foam". Carlsberg Research Laboratory. http://www.crc.dk/flab/foam.htm.
 |