Biology:Pyroptosis
Pyroptosis is a highly inflammatory form of lytic programmed cell death that occurs most frequently upon infection with intracellular pathogens and is likely to form part of the antimicrobial response. This process promotes the rapid clearance of various bacterial, viral, fungal and protozoan infections by removing intracellular replication niches and enhancing the host's defensive responses. Pyroptosis can take place in immune cells and is also reported to occur in keratinocytes and some epithelial cells.[1] The process is initiated by formation of a large supramolecular complex termed the inflammasome (also known as a pyroptosome) upon intracellular danger signals.[2] The inflammasome activates a different set of caspases as compared to apoptosis, for example, caspase-1/4/5 in humans and caspase-11 in mice.[3] These caspases contribute to the maturation and activation of the pro-inflammatory cytokines IL-1β and IL-18, as well as the pore-forming protein gasdermin D. Formation of pores causes cell membrane rupture and release of cytokines, as well as various damage-associated molecular pattern (DAMP) molecules such as HMGB-1, ATP and DNA, out of the cell. These molecules recruit more immune cells and further perpetuate the inflammatory cascade in the tissue.[4][5]
However, in pathogenic chronic diseases, the inflammatory response does not eradicate the primary stimulus. A chronic form of inflammation ensues that ultimately contributes to tissue damage. Pyroptosis is associated with diseases including autoinflammatory, metabolic, and cardiovascular diseases, as well as cancer and neurodegeneration. Some examples of pyroptosis include the cell death induced in Salmonella-infected macrophages and abortively HIV-infected T helper cells.[6][7][8]
Discovery
This type of inherently pro-inflammatory programmed cell death was named pyroptosis in 2001 by Molly Brennan and Dr. Brad T. Cookson, an associate professor of microbiology and laboratory medicine at the University of Washington.[9] The Greek pyro refers to fire and ptosis means falling. The compound term of pyroptosis may be understood as "fiery falling", which describes the bursting of pro-inflammatory chemical signals from the dying cell. Pyroptosis has a distinct morphology and mechanism compared to those of other forms of cell death.[10] It has been suggested that microbial infection was the main evolutionary pressure for this pathway.[11] Inflammasome formation was initially thought to be required for the induction of pyroptosis, but in 2013, the caspase-11 dependent noncanonical pathway was discovered, suggesting lipopolysaccharides (LPS) can trigger pyroptosis and subsequent inflammatory responses independent of toll-like receptor 4 (TLR4).[12] In 2015, gasdermin D (GSDMD) was identified as the effector of pyroptosis that forms pores in the cell membrane.[3][13] In 2021, the high-resolution structure of the GSDMD pore was solved by cryo-electron microscopy (cryo-EM).[14] Also in 2021, an additional molecule, NINJ1, was found to be required for the plasma membrane rupture during pyroptosis.[15]
Morphological characteristics
Pyroptosis, as a form of programmed cell death, has many morphological differences as compared to apoptosis. Both pyroptosis and apoptosis undergo chromatin condensation, but during apoptosis, the nucleus breaks into multiple chromatin bodies; in pyroptosis, the nucleus remains intact.[16] In a cell that undergoes pyroptosis, gasdermin pores are formed on the plasma membrane, resulting in water influx.[1][17]
In terms of mechanism, pyroptosis is activated by inflammatory caspases, including caspase-1/4/5 in humans and caspase-11 in mice. Caspase-8 can act as an upstream regulator of inflammasome activation in context-dependent manners.[18] Caspase-3 activation can take place in both apoptosis and pyroptosis.[1][17]
Although both pyroptosis and necroptosis are triggered by membrane pore formation, pyroptosis is more controlled. Cells that undergo pyroptosis exhibit membrane blebbing and produce protrusions known as pyroptotic bodies, a process not found in necroptosis.[19] Also, necroptosis works in a caspase-independent fashion. It is proposed that both pyroptosis and necroptosis may act as defence systems against pathogens when apoptotic pathways are blocked.
| Characteristics | Apoptosis | Pyroptosis | Necroptosis | |
|---|---|---|---|---|
| Morphology | Cell lysis | NO | YES | YES |
| Cell swelling | NO | YES | YES | |
| Pore formation | NO | YES | YES | |
| Membrane blebbing | YES | YES | NO | |
| DNA fragmentation | YES | YES | YES | |
| Nucleus intact | NO | YES | NO | |
| Mechanism | Caspase-1 activation | NO | YES | NO |
| Caspase-3 activation | YES | YES | NO | |
| GSDMD activation | NO | YES | NO | |
| Outcome | Inflammation | NO | YES | YES |
| Programmed cell death | YES | YES | YES |
Mechanism
The innate immune system, by using germ-line encoded pattern recognition receptors (PRRs), can recognize a wide range of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) upon microbe infection. Classic examples of PRRs include toll-like receptors (TLRs) and NOD-like receptors (NLRs).[20] Recognition of PAMPs and DAMPs triggers the formation of multi-protein complex inflammasomes, which then activates caspases to initiate pyroptosis. The inflammasome pathway may be canonical or noncanonical, with the former using caspase-1-activating inflammasomes and the latter using other caspases.[21]
The canonical inflammasome pathway
In the canonical inflammasome pathway, PAMPs and DAMPs are recognised by certain endogenous PRRs. For example, NLR proteins NLRC4 can recognise flagellin and type III secretion system components.[22] NLRP3 is activated by cellular events induced by different PAMPs and DAMPs stimuli.[23] Some non-NLR proteins like absent in melanoma 2 (AIM2) and pyrin can also be activated and form inflammasomes.[21] Also, non-inflammasome-forming PRRs such as TLRs, NOD1 and NOD2 also play important roles in pyroptosis. These receptors upregulate expression of inflammatory cytokines such as IFN α/β, tumour necrosis factor (TNF), IL-6 and IL-12 through NF-κB and MAPK-signaling pathways. In addition, pro-IL-1β and pro-IL-18 are released to be processed by cysteine-mediated caspase-1.[24][25]
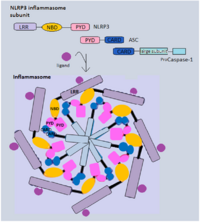
Canonical inflammasomes mostly contain three components: a sensor protein (PRRs), an adaptor (ASC) and an effector (caspase-1).[21] Generally, inflammasome-forming NLR proteins share a similar structure, several leucine-rich repeat (LRR) domains, a central nucleotide-binding and oligomerization domain (NBD) and an N-terminal pyrin domain (PYD). NLRP3, for example, recruits ASC adaptor protein via PYD-PYD interaction. Both pro-caspase-1 and ASC contain a caspase activation and recruitment domain (CARD), and this homotypic CARD-CARD interaction enables autocatalytic cleavage and reassembly of procaspase-1 to form active caspase-1.[26] Alternatively, NLRC4 can directly recruit pro-caspase-1, as it has a CARD instead of a PYD.[27] In addition to their formation as a complex to induce pyroptosis, inflammasomes can also be integral components of larger cell death-inducing complexes called PANoptosomes to induce PANoptosis, another inflammatory form of cell death.
Activated caspase-1 is responsible for cleavage of pro-IL-1β and pro-IL-18. These cytokines, once processed, will be in their biologically active form ready to be released from the host cells. In addition, caspase-1 also cleaves the cytosolic gasdermin D (GSDMD). GSDMD can be cleaved to produce an N-terminal domain (GSDMD-N) and a C-terminal domain (GSDMD-C). GSDMD-N can oligomerize and form transmembrane pores that have an inner diameter of 10-14 nm.[28] The pores allow secretion of IL-1β and IL-18 and various cytosolic content to extracellular space, and they also disrupt the cellular ionic gradient. The resulting increase in osmotic pressure causes an influx of water followed by cell swelling and bursting. Notably, GSDMD-N is autoinhibited by GSDMD C-terminal domain before cleavage to prevent cell lysis in normal conditions.[29] Also, GSDMD-N can only insert itself into the inner membrane with specific lipid compositions,[30] which limits its damage to neighbour cells. Downstream of GSDMD, NINJ1 is now thought to be required for the plasma membrane rupture during pyroptosis.[15]
The noncanonical inflammasome pathway
The noncanonical inflammasome pathway is initiated by binding of lipopolysaccharide (LPS) of gram-negative bacteria directly onto caspase-4/5 in humans and caspase-11 in murines. Binding of LPS onto these caspases promotes their oligomerization and activation.[12] These caspases can cleave GSDMD to release GSDMD-N and trigger pyroptosis. In addition, an influx of potassium ions upon membrane permeabilization triggers activation of NLRP3, which then leads to formation of NLRP3 inflammasome and activation of caspase-1.[21] These processes facilitate the cleavage of GSDMD and promote the maturation and release of pro-inflammatory cytokines.

Caspase-3-dependent cell death pathway
An alternative pathway that links apoptosis and pyroptosis has been recently proposed. Caspase-3, an executioner caspase in apoptosis, can cleave gasdermin E (GSDME) to produce a N-terminal fragment and a C-terminal fragment in a way similar to GSDMD cleavage.[3] When apoptotic cells are not scavenged by macrophages, GSDME expression is then upregulated by p53. GSDME is then activated by caspase-3 to form pores on the cell membrane. It has also been found that GSDME can permeabilise mitochondrial membranes to release cytochrome c, which further activates caspase-3 and accelerates GSDME cleavage.[31] This positive feedback loop ensures that programmed cell death is carried forward.
Clinical relevance
Infection
Pyroptosis acts as a defence mechanism against infection by inducing pathological inflammation. The formation of inflammasomes and the activity of caspase-1 determine the balance between pathogen resolution and disease.
In a healthy cell, caspase-1 activation helps to fight infection caused by Salmonella and Shigella by introducing cell death to restrict pathogen growth.[6] When the "danger" signal is sensed, the quiescent cells will be activated to undergo pyroptosis and produce inflammatory cytokines IL-1β and IL-18. IL-18 will stimulate IFNγ production and initiates the development of TH1 responses. (TH1 responses tend to release cytokines that direct an immediate removal of the pathogen.)[32] The cell activation results in an increase in cytokine levels, which will augment the consequences of inflammation and this, in turn, contributes to the development of the adaptive response as infection progresses. The ultimate resolution will clear pathogens.
In contrast, persistent inflammation will produce excessive immune cells, which is detrimental. If the amplification cycles persist, metabolic disorder, autoinflammatory diseases and liver injury associated with chronic inflammation will occur.[32]
Recently, pyroptosis and downstream pathways were identified as promising targets for treatment of severe COVID-19-associated diseases.[33]
Cerebrovascular disease
Recent studies show that pyroptosis plays a role in the pathophysiology of intracerebral hemorrhage, and mitigating pyroptosis could be an intervention strategy to inhibit the inflammatory response after intracerebral hemorrhage.[34]
Cancer
Pyroptosis, as an inflammation-associated programmed cell death, has wide implications in various cancer types. Principally, pyroptosis can kill cancer cells and inhibit tumour development in the presence of endogenous DAMPs. In some cases, GSDMD can be used as a prognostic marker for cancers. However, prolonged production of inflammatory bodies may facilitate the formation of microenvironments that favour tumour growth.[35] Understanding the mechanisms of pyroptosis and identifying pyroptosis-associated molecules can be useful in treating different cancers.
In gastric cancer cells, presence of GSDMD can inhibit cyclin A2/CDK2 complexes, leading to cell cycle arrest and thus inhibit tumour development. Also, cellular concentration of GSDME increases when gastric cancer cells are treated with certain chemotherapy drugs. GSDME then activates caspase-3 and triggers pyroptotic cell death.[17]
Cervical cancer can be caused by human papillomavirus (HPV) infection. AIM2 protein can recognise viral DNA in cytoplasm and form AIM2 inflammasome, which then triggers by a caspase-1 dependent canonical pyroptosis pathway. HPV infection causes the upregulation of sirtuin 1 protein, which disrupts the transcription factor for AIM2, RelB. Knockdown of sirtuin 1 upregulates AIM2 expression and triggers pyroptosis.[36]
Metabolic disorder
The level of expression of NLRP3 inflammasome and caspase-1 has a direct relation to the severity of several metabolic syndromes, such as obesity and type II diabetic mellitus (T2DM). This is because the subsequent production level of IL-1β and IL-18, cytokines that impair the secretion of insulin, is affected by the activity of caspase-1. Glucose uptake level is then diminished, and the condition is known as insulin resistance.[37] The condition is further accelerated by the IL-1β-induced destruction of pancreatic β cells.[38]
Cryopyrinopathies
A mutation in the gene coding of inflammasomes leads to a group of autoinflammatory diseases called cryopyrinopathies. This group includes Muckle–Wells syndrome, cold autoinflammatory syndrome and chronic infantile neurologic cutaneous and articular syndrome, all showing symptoms of sudden fevers and localized inflammation.[39] The mutated gene in such cases is the NLRP3, impeding the activation of inflammasome and resulting in an excessive production of IL-1β. This effect is known as "gain-of-function".[40]
HIV and AIDS
Recent studies demonstrate that caspase-1-mediated pyroptosis drives CD4 T-cell depletion and inflammation by HIV,[7][41][42][43] two signature events that propel HIV disease progression to AIDS. Although pyroptosis contributes to the host's ability to rapidly limit and clear infection by removing intracellular replication niches and enhancing defensive responses through the release of proinflammatory cytokines and endogenous danger signals, in pathogenic inflammation, such as that elicited by HIV-1, this beneficial response does not eradicate the primary stimulus. In fact, it appears to create a pathogenic vicious cycle in which dying CD4 T cells release inflammatory signals that attract more cells into the infected lymphoid tissues to die and to produce chronic inflammation and tissue injury. It may be possible to break this pathogenic cycle with safe and effective caspase-1 inhibitors. These agents could form a new and exciting 'anti-AIDS' therapy for HIV-infected subjects in which the treatment targets the host instead of the virus. Of note, Caspase-1 deficient mice develop normally,[44][45] arguing that inhibition of this protein would produce beneficial rather than harmful therapeutic effects in HIV patients.
References
- ↑ 1.0 1.1 1.2 "Pyroptotic cell death defends against intracellular pathogens". Immunological Reviews 265 (1): 130–42. May 2015. doi:10.1111/imr.12287. PMID 25879289.
- ↑ "Cell death mechanisms in eukaryotes". Cell Biology and Toxicology 36 (2): 145–164. April 2020. doi:10.1007/s10565-019-09496-2. PMID 31820165.
- ↑ 3.0 3.1 3.2 "Research progresses of molecular mechanism of pyroptosis and its related diseases". Immunobiology 225 (2): 151884. March 2020. doi:10.1016/j.imbio.2019.11.019. PMID 31822435.
- ↑ "The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response". Nature Immunology 15 (8): 738–48. August 2014. doi:10.1038/ni.2919. PMID 24952504.
- ↑ "The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation". Nature Immunology 15 (8): 727–37. August 2014. doi:10.1038/ni.2913. PMID 24952505.
- ↑ 6.0 6.1 6.2 "Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages". Cellular Microbiology 8 (11): 1812–25. November 2006. doi:10.1111/j.1462-5822.2006.00751.x. PMID 16824040.
- ↑ 7.0 7.1 "Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection". Nature 505 (7484): 509–14. January 2014. doi:10.1038/nature12940. PMID 24356306. Bibcode: 2014Natur.505..509D.
- ↑ "Dissecting How CD4 T Cells Are Lost During HIV Infection". Cell Host & Microbe 19 (3): 280–91. March 2016. doi:10.1016/j.chom.2016.02.012. PMID 26962940.
- ↑ "Pro-inflammatory programmed cell death". Trends in Microbiology 9 (3): 113–4. March 2001. doi:10.1016/S0966-842X(00)01936-3. PMID 11303500.
- ↑ 10.0 10.1 "Major cell death pathways at a glance". Microbes and Infection 11 (13): 1050–62. November 2009. doi:10.1016/j.micinf.2009.08.013. PMID 19733681.
- ↑ "The inflammasome: in memory of Dr. Jurg Tschopp". Cell Death and Differentiation 19 (1): 5–12. January 2012. doi:10.1038/cdd.2011.159. PMID 22075986.
- ↑ 12.0 12.1 "Inflammatory caspases are innate immune receptors for intracellular LPS". Nature 514 (7521): 187–92. October 2014. doi:10.1038/nature13683. PMID 25119034. Bibcode: 2014Natur.514..187S.
- ↑ "Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death". Nature 526 (7575): 660–5. October 2015. doi:10.1038/nature15514. PMID 26375003. Bibcode: 2015Natur.526..660S.
- ↑ Xia, Shiyu; Zhang, Zhibin; Magupalli, Venkat Giri; Pablo, Juan Lorenzo; Dong, Ying; Vora, Setu M.; Wang, Longfei; Fu, Tian-Min et al. (2021-04-21). "Gasdermin D pore structure reveals preferential release of mature interleukin-1" (in en). Nature 593 (7860): 607–611. doi:10.1038/s41586-021-03478-3. ISSN 0028-0836. PMID 33883744. Bibcode: 2021Natur.593..607X.
- ↑ 15.0 15.1 Kayagaki, Nobuhiko (2021-01-20). "NINJ1 mediates plasma membrane rupture during lytic cell death" (in en). Nature 591 (7848): 131–136. doi:10.1038/s41586-021-03218-7. PMID 33472215. Bibcode: 2021Natur.591..131K.
- ↑ "Salmonella induces macrophage death by caspase-1-dependent necrosis". Molecular Microbiology 38 (1): 31–40. October 2000. doi:10.1046/j.1365-2958.2000.02103.x. PMID 11029688.
- ↑ 17.0 17.1 17.2 17.3 "Pyroptosis: A new frontier in cancer". Biomedicine & Pharmacotherapy 121: 109595. January 2020. doi:10.1016/j.biopha.2019.109595. PMID 31710896.
- ↑ "FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes". Journal of Immunology 192 (4): 1835–1846. January 22, 2014. doi:10.4049/jimmunol.1302839. PMID 24453255.
- ↑ "Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis". Cell Research 26 (9): 1007–20. September 2016. doi:10.1038/cr.2016.100. PMID 27573174.
- ↑ "Control of infection by pyroptosis and autophagy: role of TLR and NLR". Cellular and Molecular Life Sciences 67 (10): 1643–51. May 2010. doi:10.1007/s00018-010-0335-5. PMID 20229126.
- ↑ 21.0 21.1 21.2 21.3 "NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways". Archives of Biochemistry and Biophysics 670: 4–14. July 2019. doi:10.1016/j.abb.2019.02.008. PMID 30772258.
- ↑ "The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus". Nature 477 (7366): 596–600. September 2011. doi:10.1038/nature10510. PMID 21918512. Bibcode: 2011Natur.477..596Z.
- ↑ "The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation". International Journal of Molecular Sciences 20 (13): 3328. July 2019. doi:10.3390/ijms20133328. PMID 31284572.
- ↑ "TLR signaling". Cell Death and Differentiation 13 (5): 816–25. May 2006. doi:10.1038/sj.cdd.4401850. PMID 16410796.
- ↑ "NOD1 and NOD2 in inflammation, immunity and disease". Archives of Biochemistry and Biophysics 670: 69–81. July 2019. doi:10.1016/j.abb.2018.12.022. PMID 30578751.
- ↑ "Inflammasomes: mechanism of assembly, regulation and signalling". Nature Reviews. Immunology 16 (7): 407–20. July 2016. doi:10.1038/nri.2016.58. PMID 27291964.
- ↑ "The NLRC4 Inflammasome". Immunological Reviews 281 (1): 115–123. January 2018. doi:10.1111/imr.12607. PMID 29247997.
- ↑ "Pore-forming activity and structural autoinhibition of the gasdermin family". Nature 535 (7610): 111–6. July 2016. doi:10.1038/nature18590. PMID 27281216. Bibcode: 2016Natur.535..111D.
- ↑ "Structures of the Gasdermin D C-Terminal Domains Reveal Mechanisms of Autoinhibition". Structure 26 (5): 778–784.e3. May 2018. doi:10.1016/j.str.2018.03.002. PMID 29576317.
- ↑ "'Hints' in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death". Cell Death and Differentiation 24 (4): 588–596. April 2017. doi:10.1038/cdd.2017.24. PMID 28362726.
- ↑ "Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation". Nature Communications 10 (1): 1689. April 2019. doi:10.1038/s41467-019-09397-2. PMID 30976076. Bibcode: 2019NatCo..10.1689R.
- ↑ 32.0 32.1 "The inflammasome NLRs in immunity, inflammation, and associated diseases". Annual Review of Immunology 29 (1): 707–35. 2011. doi:10.1146/annurev-immunol-031210-101405. PMID 21219188.
- ↑ Yap, Jeremy K. Y.; Moriyama, Miyu; Iwasaki, Akiko (2020-07-15). "Inflammasomes and Pyroptosis as Therapeutic Targets for COVID-19". Journal of Immunology 205 (2): 307–312. doi:10.4049/jimmunol.2000513. ISSN 1550-6606. PMID 32493814.
- ↑ "Perspectives on the mechanism of pyroptosis after intracerebral hemorrhage". Front Immunol 2022: 989503. September 2022. doi:10.3389/fimmu.2022.989503. PMID 36131917.
- ↑ "The role of pyroptosis in cancer: pro-cancer or pro-"host"?". Cell Death & Disease 10 (9): 650. September 2019. doi:10.1038/s41419-019-1883-8. PMID 31501419.
- ↑ "Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense". Oncogene 37 (38): 5191–5204. September 2018. doi:10.1038/s41388-018-0339-4. PMID 29844574.
- ↑ "The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance". Nature Medicine 17 (2): 179–88. February 2011. doi:10.1038/nm.2279. PMID 21217695.
- ↑ "Inflammasomes in health and disease". Nature 481 (7381): 278–86. January 2012. doi:10.1038/nature10759. PMID 22258606. Bibcode: 2012Natur.481..278S.
- ↑ "Cryopyrinopathies: update on pathogenesis and treatment". Nature Clinical Practice. Rheumatology 4 (9): 481–9. September 2008. doi:10.1038/ncprheum0874. PMID 18665151.
- ↑ "Primer: inflammasomes and interleukin 1beta in inflammatory disorders". Nature Clinical Practice. Rheumatology 4 (1): 34–42. January 2008. doi:10.1038/ncprheum0681. PMID 18172447.
- ↑ "IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV". Science 343 (6169): 428–32. January 2014. doi:10.1126/science.1243640. PMID 24356113. Bibcode: 2014Sci...343..428M.
- ↑ Lu, Wuxun; Demers, Andrew J.; Ma, Fangrui; Kang, Guobin; Yuan, Zhe; Wan, Yanmin; Li, Yue; Xu, Jianqing et al. (2016-01-15). Silvestri, G.. ed. "Next-Generation mRNA Sequencing Reveals Pyroptosis-Induced CD4 + T Cell Death in Early Simian Immunodeficiency Virus-Infected Lymphoid Tissues" (in en). Journal of Virology 90 (2): 1080–1087. doi:10.1128/JVI.02297-15. ISSN 0022-538X. PMID 26559826.
- ↑ Zhang, Chao; Song, Jin-Wen; Huang, Hui-Huang; Fan, Xing; Huang, Lei; Deng, Jian-Ning; Tu, Bo; Wang, Kun et al. (2021-03-15). "NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1–infected patients" (in en). The Journal of Clinical Investigation 131 (6). doi:10.1172/JCI138861. ISSN 0021-9738. PMID 33720048.
- ↑ "Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme". Science 267 (5206): 2000–3. March 1995. doi:10.1126/science.7535475. PMID 7535475. Bibcode: 1995Sci...267.2000K.
- ↑ "Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock". Cell 80 (3): 401–11. February 1995. doi:10.1016/0092-8674(95)90490-5. PMID 7859282.
 |
