Biology:Radial glial cell
| Radial glial cell | |
|---|---|
 | |
| Details | |
| Identifiers | |
| Latin | gliocytus radialis |
| Anatomical terms of microanatomy | |
Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes.[2][3][4] Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.
During development, newborn neurons use radial glia as scaffolds, traveling along the radial glial fibers in order to reach their final destinations.[3][5][6] Despite the various possible fates of the radial glial population, it has been demonstrated through clonal analysis that most radial glia have restricted, unipotent or multipotent, fates. Radial glia can be found during the neurogenic phase in all vertebrates (studied to date).[7]
The term "radial glia" refers to the morphological characteristics of these cells that were first observed: namely, their radial processes and their similarity to astrocytes, another member of the glial cell family.[8]
Structure
Müller glia
Müller glia are radial glial cells that are present in the developing, as well as the adult, retina. As in the cortex, Müller glia have long processes that span the entire width of the retina, from the basal cell layer to the apical layer. However, unlike cortical radial glia, Müller glia do not appear in the retina until after the first rounds of neurogenesis have occurred. Studies suggest that Müller glia can dedifferentiate into readily dividing neural progenitors in response to injury.[9]
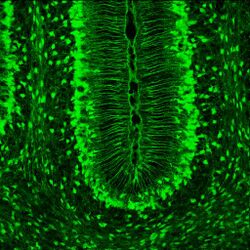
The characteristics that truly set Müller glia apart from radial glia in other areas of the brain, is their possession of optical properties. The majority of the retina is actually largely light scattering, suggesting that Müller glia serve as the main fiber responsible for the relay of light to the photoreceptors in the rear of the retina. Properties that help Müller glia achieve this function include a limited number mitochondria (which are very light scattering), as well as a specialized arrangement of internal protein filaments.[9]
Müller glia are the predominant type of macroglia in the retina, so they take on many of the supportive functions that astrocytes and oligodendrocytes usually handle in the rest of the central nervous system.[9]
Bergmann glia

Bergmann glia (also known as radial epithelial cells, Golgi epithelial cells, or radial astrocytes) are unipolar astrocytes derived from radial glia that are intimately associated with Purkinje cells in the cerebellum.[10] Since bergmann glia appear to persist in the cerebellum, and perform many of the roles characteristic of astrocytes, they have also been called "specialized astrocytes."[9] Bergmann glia have multiple radial processes that extend across the molecular layer of the cerebellar cortex and terminate at the pial surface as a bulbous endfoot.[11] Bergmann glial cells assist with the migration of granule cells, guiding the small neurons from the external granular layer down to the internal granular layer along their extensive radial processes.[12][13] Besides their role in early development of the cerebellum, Bergmann glia are also required for synaptic pruning.[14] Following Purkinje cell death induced by CNS injury, Bergmann glia undergo extensive proliferative changes so as to replace lost or damaged tissue in a process known as gliosis.[15][16]
Development
Radial glial cells originate from the transformation of neuroepithelial cells that form the neural plate during neurogenesis in early embryonic development.[8][9][17] This process is mediated through the down-regulation of epithelium-related protein expression (such as tight junctions) and an up-regulation of glial-specific features such as glycogen granules, the astrocyte glutamate aspartate transporter (GLAST), the intermediate filament vimentin, and, in some instances, including humans, glial fibrillary acidic protein (GFAP).[7]
After this transition, radial glia retain many of the original characteristics of neuroepithelial cells including: their apical-basal polarity, their position along the lateral ventricles of the developing cortex, and the phasic migration of their nuclei depending on their location with the cell cycle (termed “interkinetic nuclear migration”).[9][18][19]
Function
Progenitors
Radial glia are now recognized as key progenitor cells in the developing nervous system. During the late stages of neurogenesis, radial glial cells divide asymmetrically in the ventricular zone, generating a new radial glial cell, as well as a postmitotic neuron or an intermediate progenitor (IPC) daughter cell. Intermediate progenitor cells then divide symmetrically in the subventricular zone to generate neurons.[18] Local environmental cues such as Notch and fibroblast growth factor (FGF) signaling, developmental period, and differing abilities of radial glia to respond to environmental cues have all been shown to influence the type of radial glia and radial glia-derived daughter cells that will be produced. FGF and Notch signaling regulate the proliferation of radial glia and the rate of neurogenesis, which affects the surface area expansion of the cerebral cortex and its ability to form surface convolutions known as gyri (see gyrification).[9][20][21] Radial glial cells show high levels of calcium transient activity, which is transmitted between RGCs in the ventricular zone and along the radial fibers bidirectionally to/from the cortical plate.[22][23] The calcium activity is thought to promote RGC proliferation and could be involved in radial communication before synapses are present in the brain. Additionally, recent evidence suggests that cues from the external sensory environment can also influence the proliferation and neural differentiation of radial glia.[9][24]
At the conclusion of cortical development, most radial glia lose their attachment to the ventricles, and migrate towards the surface of the cortex, where, in mammals, most will become astrocytes during the process of gliogenesis.[18]
While it has been suggested that radial glia most likely give rise to oligodendrocytes, through the generation of oligodendrocyte progenitor cells (OPCs), and OPCs can be generated from radial glial cells in vitro, more evidence is yet needed to conclude whether this process also occurs in the developing brain.[18][25]
Recently, radial glia that exclusively generate upper-layer cortical neurons have also been discovered.[8] Since upper cortical layers have expanded greatly in recent evolution, and are associated with higher-level information processing and thinking, radial glia have been implicated as important mediators of brain evolution.[26]
Migration Pattern
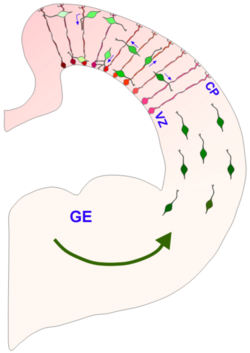
The best characterized and first widely accepted function of radial glia is their role as scaffolds for neuronal migration in the cerebral and cerebellar cortexes. This role can be easily visualized using the electron microscope or high-resolution time-lapse microscopy, through which neurons can be seen tightly wrapped around radial glia as they travel upwards through the cortex.[8] Additional evidence suggests that many neurons may move between neighboring radial glial fibers during migration.[9]
While excitatory neuronal migration is largely radial, inhibitory, GABAergic neurons have been shown to undergo tangential migration. Tangentially migrating neurons also appear to initiate contact with radial glial fibers in the developing cortex of ferrets, implicating radial glial cells in both of these forms of migration.[9]
As radial glia seem to differentiate late in spinal cord development, near the onset of gliogenesis, it is unclear whether they are involved in spinal cord neurogenesis or migration.[8]
Compartmentalization
Radial glia have also been implicated in forming boundaries between different axonal tracts and white matter areas of the brain.[8][27]
Clinical significance
As radial glia serve as the primary neural and glial progenitors in the brain, as well as being crucial for proper neuronal migration, defects in radial glial function can have profound effects in the development of the nervous system.
Mutations in either Lis1 or Nde1, essential proteins for radial glial differentiation and stabilization, cause the associated neurodevelopmental diseases Lissencephaly and microlissencephaly (which literally translate to “smooth brain”). Patients with these diseases are characterized by a lack of cortical folds (sulci and gyri) and reduced brain volume. Extreme cases of Lissencephaly cause death a few months after birth, while patients with milder forms may experience mental retardation, difficulty balancing, motor and speech deficits, and epilepsy.[8]
Death of neural progenitor cells has recently been linked the mosquito-borne virus, Zika.[28] Epidemiological evidence indicates infection of the embryo within the first two trimesters of pregnancy has potential to cause fetal birth defects and microcephaly,[29] possibly due to the death of progenitor cells. Further, mutations in microcephaly associated genes which encode proteins such as WDR62 can lead to radial glial depletion during brain development which ultimately leads to a smaller brain size and mental disabilities. [30]
History
Camillo Golgi, using his silver staining technique (later deemed the Golgi method), first described radially oriented cells spanning from the central canal to the outer surface of the embryonic chick spinal cord, in 1885.[31]
Using the Golgi method, Giuseppe Magini then studied the mammalian fetal cerebral cortex in 1888, confirming the similar presence of elongated radial cells in the cortex (also described by Kölliker just before him), and observing “various varicosities or swellings” on the radial fibers. Intrigued, Magini also observed that the size and number of these varicosities increased later in development, and were absent in the adult nervous system. Based on these findings, Magini then hypothesized that these varicosities could be developing neurons. Using a combination Golgi and hematoxylin staining method, Magini was able to identify these varicosities as cells, some of which were very closely associated with the radial fibers.[31]
Additional early works that were important in elucidating the identity and function of radial glia, were completed by Ramón y Cajal, who first suggested that the radial cells were a type of glia through their similarities to astrocytes;[8] and Wilhelm His, who also proposed the idea that growing axons may use radial cells for orientation and guidance during development.[31]
Despite the initial period of interest in radial glia, little additional information was learned about these cells until the electron microscope and immunohistochemistry became available some 60 years later.[31]
See also
List of distinct cell types in the adult human body
References
- ↑ "The receptor for granulocyte-colony stimulating factor (G-CSF) is expressed in radial glia during development of the nervous system". BMC Developmental Biology 8: 32. March 2008. doi:10.1186/1471-213X-8-32. PMID 18371196.
- ↑ Beattie, R; Hippenmeyer, S (December 2017). "Mechanisms of radial glia progenitor cell lineage progression.". FEBS Letters 591 (24): 3993–4008. doi:10.1002/1873-3468.12906. PMID 29121403.
- ↑ 3.0 3.1 "Evolution of the neocortex: a perspective from developmental biology". Nature Reviews. Neuroscience 10 (10): 724–35. October 2009. doi:10.1038/nrn2719. PMID 19763105.
- ↑ "Neurons derived from radial glial cells establish radial units in neocortex". Nature 409 (6821): 714–20. February 2001. doi:10.1038/35055553. PMID 11217860. Bibcode: 2001Natur.409..714N.
- ↑ "Mode of cell migration to the superficial layers of fetal monkey neocortex". The Journal of Comparative Neurology 145 (1): 61–83. May 1972. doi:10.1002/cne.901450105. PMID 4624784.
- ↑ "Conservation of neural progenitor identity and the emergence of neocortical neuronal diversity". Seminars in Cell and Developmental Biology 118 (118): 4–13. October 2021. doi:10.1016/j.semcdb.2021.05.024. PMID 34083116. https://www.sciencedirect.com/science/article/abs/pii/S1084952121001336.
- ↑ 7.0 7.1 "Radial glia and neural stem cells". Cell and Tissue Research 331 (1): 165–78. January 2008. doi:10.1007/s00441-007-0481-8. PMID 17846796.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 "Radial glial cells: key organisers in CNS development". The International Journal of Biochemistry & Cell Biology 46: 76–9. January 2014. doi:10.1016/j.biocel.2013.11.013. PMID 24269781.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 "Radial glia: progenitor, pathway, and partner". The Neuroscientist 17 (3): 288–302. June 2011. doi:10.1177/1073858410385870. PMID 21558559.
- ↑ Verkhratsky, Alexei; Butt, Arthur M. (2013). Glial Physiology and Pathophysiology. John Wiley and Sons, Inc.. ISBN 9780470978535.
- ↑ "The monolayer formation of Bergmann glial cells is regulated by Notch/RBP-J signaling". Developmental Biology 311 (1): 238–50. November 2007. doi:10.1016/j.ydbio.2007.08.042. PMID 17915208.
- ↑ Rubenstein, John; Rakic, Pasko (2013). Cellular Migration and Formation of Neuronal Connections: Comprehensive Developmental Neuroscience. Elsevier Science and Technology. ISBN 9780123972668.
- ↑ Sanes, Dan H.; Reh, Thomas A.; Harris, William A. (2005). Development of the Nervous System. Elsevier Science and Technology. ISBN 9780126186215.
- ↑ "Bergmann Glial Cell". 14 Oct 2011. http://neurolex.org/wiki/Category:Bergmann_Glial_Cell.
- ↑ "Astrogliosis". Cold Spring Harbor Perspectives in Biology 7 (2): a020420. November 2014. doi:10.1101/cshperspect.a020420. PMID 25380660.
- ↑ Catherine., Haberland (2007). Clinical neuropathology : text and color atlas. New York: Demos. ISBN 9781934559529. OCLC 166267295.
- ↑ "Conservation of neural progenitor identity and the emergence of neocortical neuronal diversity". Seminars in Cell and Developmental Biology 118 (118): 4–13. October 2021. doi:10.1016/j.semcdb.2021.05.024. PMID 34083116. https://www.sciencedirect.com/science/article/abs/pii/S1084952121001336.
- ↑ 18.0 18.1 18.2 18.3 "The glial nature of embryonic and adult neural stem cells". Annual Review of Neuroscience 32: 149–84. 2009. doi:10.1146/annurev.neuro.051508.135600. PMID 19555289.
- ↑ "Conservation of neural progenitor identity and the emergence of neocortical neuronal diversity". Seminars in Cell and Developmental Biology 118 (118): 4–13. October 2021. doi:10.1016/j.semcdb.2021.05.024. PMID 34083116. https://www.sciencedirect.com/science/article/abs/pii/S1084952121001336.
- ↑ "FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis". The Journal of Neuroscience 31 (43): 15604–17. October 2011. doi:10.1523/jneurosci.4439-11.2011. PMID 22031906.
- ↑ "Cortical gyrification induced by fibroblast growth factor 2 in the mouse brain". The Journal of Neuroscience 33 (26): 10802–14. June 2013. doi:10.1523/jneurosci.3621-12.2013. PMID 23804101.
- ↑ "Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex". Neuron 43 (5): 647–61. September 2004. doi:10.1016/j.neuron.2004.08.015. PMID 15339647.
- ↑ "Bidirectional radial Ca(2+) activity regulates neurogenesis and migration during early cortical column formation". Science Advances 2 (2): e1501733. February 2016. doi:10.1126/sciadv.1501733. PMID 26933693. Bibcode: 2016SciA....2E1733R.
- ↑ "Visual activity regulates neural progenitor cells in developing xenopus CNS through musashi1". Neuron 68 (3): 442–55. November 2010. doi:10.1016/j.neuron.2010.09.028. PMID 21040846.
- ↑ "Human fetal radial glia cells generate oligodendrocytes in vitro". Glia 57 (5): 490–8. April 2009. doi:10.1002/glia.20775. PMID 18814269.
- ↑ "Scripps Research Neuroscientists Find Brain Stem Cells that May Be Responsible for Higher Functions, Bigger Brains". Scripps Research Institute. http://www.scripps.edu/news/press/2012/20120809mueller.html.
- ↑ "Glial boundaries in the developing nervous system". Annual Review of Neuroscience 16: 445–70. 1993. doi:10.1146/annurev.ne.16.030193.002305. PMID 8460899.
- ↑ "Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth". Cell Stem Cell 18 (5): 587–90. May 2016. doi:10.1016/j.stem.2016.02.016. PMID 26952870.
- ↑ "Zika Virus Associated with Microcephaly". The New England Journal of Medicine 374 (10): 951–8. March 2016. doi:10.1056/NEJMoa1600651. PMID 26862926.
- ↑ "The association of microcephaly protein WDR62 with CPAP/IFT88 is required for cilia formation and neocortical development". Human Molecular Genetics 29 (2): 248–263. January 2020. doi:10.1093/hmg/ddz281. PMID 31816041.
- ↑ 31.0 31.1 31.2 31.3 "The history of radial glia". Brain Research Bulletin 49 (5): 305–15. July 1999. doi:10.1016/s0361-9230(99)00065-9. PMID 10452351.
 |
