Biology:Glial fibrillary acidic protein
 Generic protein structure example |
Glial fibrillary acidic protein (GFAP) is a protein that is encoded by the GFAP gene in humans.[1] It is a type III intermediate filament (IF) protein that is expressed by numerous cell types of the central nervous system (CNS), including astrocytes[2] and ependymal cells during development.[3] GFAP has also been found to be expressed in glomeruli and peritubular fibroblasts taken from rat kidneys,[4] Leydig cells of the testis in both hamsters[5] and humans,[6] human keratinocytes,[7] human osteocytes and chondrocytes[8] and stellate cells of the pancreas and liver in rats.[9]
GFAP is closely related to the other three non-epithelial type III IF family members, vimentin, desmin and peripherin, which are all involved in the structure and function of the cell's cytoskeleton. GFAP is thought to help to maintain astrocyte mechanical strength[10] as well as the shape of cells, but its exact function remains poorly understood, despite the number of studies using it as a cell marker. The protein was named and first isolated and characterized by Lawrence F. Eng in 1969.[11] In humans, it is located on the long arm of chromosome 17.[12]
Structure
Type III intermediate filaments contain three domains, named the head, rod and tail domains. The specific DNA sequence for the rod domain may differ between different type III intermediate filaments, but the structure of the protein is highly conserved. This rod domain coils around that of another filament to form a dimer, with the N-terminal and C-terminal of each filament aligned. Type III filaments such as GFAP are capable of forming both homodimers and heterodimers; GFAP can polymerize with other type III proteins.[13] GFAP and other type III IF proteins cannot assemble with keratins, the type I and II intermediate filaments: in cells that express both proteins, two separate intermediate filament networks form,[14] which can allow for specialization and increased variability.
To form networks, the initial GFAP dimers combine to make staggered tetramers,[15] which are the basic subunits of an intermediate filament. Since rod domains alone in vitro do not form filaments, the non-helical head and tail domains are necessary for filament formation.[13] The head and tail regions have greater variability of sequence and structure. In spite of this increased variability, the head of GFAP contains two conserved arginines and an aromatic residue that have been shown to be required for proper assembly.[16]
Function in the central nervous system
GFAP is expressed in the central nervous system in astrocyte cells, and the concentration of GFAP differs between different regions in the CNS, where the highest levels are found in medulla oblongata, cervical spinal cord and hippocampus.[2][17][18] It is involved in many important CNS processes, including cell communication and the functioning of the blood brain barrier.
GFAP has been shown to play a role in mitosis by adjusting the filament network present in the cell. During mitosis, there is an increase in the amount of phosphorylated GFAP, and a movement of this modified protein to the cleavage furrow.[19] There are different sets of kinases at work; cdc2 kinase acts only at the G2 phase transition, while other GFAP kinases are active at the cleavage furrow alone. This specificity of location allows for precise regulation of GFAP distribution to the daughter cells. Studies have also shown that GFAP knockout mice undergo multiple degenerative processes including abnormal myelination, white matter structure deterioration, and functional/structural impairment of the blood–brain barrier.[20] These data suggest that GFAP is necessary for many critical roles in the CNS.
GFAP is proposed to play a role in astrocyte-neuron interactions as well as cell-cell communication. In vitro, using antisense RNA, astrocytes lacking GFAP do not form the extensions usually present with neurons.[21] Studies have also shown that Purkinje cells in GFAP knockout mice do not exhibit normal structure, and these mice demonstrate deficits in conditioning experiments such as the eye-blink task.[22] Biochemical studies of GFAP have shown MgCl2 and/or calcium/calmodulin dependent phosphorylation at various serine or threonine residues by PKC and PKA[23] which are two kinases that are important for the cytoplasmic transduction of signals. These data highlight the importance of GFAP for cell-cell communication.
GFAP has also been shown to be important in repair after CNS injury. More specifically for its role in the formation of glial scars in a multitude of locations throughout the CNS including the eye[24] and brain.[25]
Autoimmune GFAP astrocytopathy
In 2016 a CNS inflammatory disorder associated with anti-GFAP antibodies was described. Patients with autoimmune GFAP astrocytopathy developed meningoencephalomyelitis with inflammation of the meninges, the brain parenchyma, and the spinal cord. About one third of cases were associated with various cancers and many also expressed other CNS autoantibodies.
Meningoencephalitis is the predominant clinical presentation of autoimmune GFAP astrocytopathy in published case series.[26] It also can appear associated with encephalomyelitis and parkinsonism.[27]
Disease states

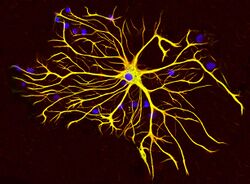
There are multiple disorders associated with improper GFAP regulation, and injury can cause glial cells to react in detrimental ways. Glial scarring is a consequence of several neurodegenerative conditions, as well as injury that severs neural material. The scar is formed by astrocytes interacting with fibrous tissue to re-establish the glial margins around the central injury core[28] and is partially caused by up-regulation of GFAP.[29]
Another condition directly related to GFAP is Alexander disease, a rare genetic disorder. Its symptoms include mental and physical retardation, dementia, enlargement of the brain and head, spasticity (stiffness of arms and/or legs), and seizures.[30] The cellular mechanism of the disease is the presence of cytoplasmic accumulations containing GFAP and heat shock proteins, known as Rosenthal fibers.[31] Mutations in the coding region of GFAP have been shown to contribute to the accumulation of Rosenthal fibers.[32] Some of these mutations have been proposed to be detrimental to cytoskeleton formation as well as an increase in caspase 3 activity,[33] which would lead to increased apoptosis of cells with these mutations. GFAP therefore plays an important role in the pathogenesis of Alexander disease.
Notably, the expression of some GFAP isoforms have been reported to decrease in response to acute infection or neurodegeneration.[34] Additionally, reduction in GFAP expression has also been reported in Wernicke's encephalopathy.[35] The HIV-1 viral envelope glycoprotein gp120 can directly inhibit the phosphorylation of GFAP and GFAP levels can be decreased in response to chronic infection with HIV-1,[36] varicella zoster,[37] and pseudorabies.[38] Decreases in GFAP expression have been reported in Down's syndrome, schizophrenia, bipolar disorder and depression.[34]
The generally high abundance of GFAP in the CNS has led to a great interest in GFAP as a blood biomarker of acute injury to the brain and spinal cord in different types of disease mechanisms, such as traumatic brain injury and cerebrovascular disease.[39] Elevated blood levels of GFAP are also found in neuroinflammatory diseases, such as multiple sclerosis and neuromyelitis optica, a disease targeting astrocytes.[39] In a study of 22 child patients undergoing extracorporeal membrane oxygenation (ECMO), children with abnormally high levels of GFAP were 13 times more likely to die and 11 times more likely to suffer brain injury than children with normal GFAP levels.[40]
Interactions
Glial fibrillary acidic protein has been shown to interact with MEN1[41] and PSEN1.[42]
Isoforms
Although GFAP alpha is the only isoform which is able to assemble homomerically, GFAP has 8 different isoforms which label distinct subpopulations of astrocytes in the human and rodent brain. These isoforms include GFAP kappa, GFAP +1 and the currently best researched GFAP delta. GFAP delta appears to be linked with neural stem cells (NSCs) and may be involved in migration. GFAP+1 is an antibody which labels two isoforms. Although GFAP+1 positive astrocytes are supposedly not reactive astrocytes, they have a wide variety of morphologies including processes of up to 0.95mm (seen in the human brain). The expression of GFAP+1 positive astrocytes is linked with old age and the onset of AD pathology.[43]
See also
- 17q21.31 microdeletion syndrome (Koolen–de Vries syndrome)
- GFAP stain
References
- ↑ "Determination of the gene structure of human GFAP and absence of coding region mutations associated with frontotemporal dementia with parkinsonism linked to chromosome 17". Genomics 51 (1): 152–154. July 1998. doi:10.1006/geno.1998.5360. PMID 9693047.
- ↑ 2.0 2.1 "Determination of glial fibrillary acidic protein (GFAP) in human brain tumors". Journal of the Neurological Sciences 35 (1): 147–155. January 1978. doi:10.1016/0022-510x(78)90107-7. PMID 624958.
- ↑ "Glial fibrillary acidic protein (GFAP) in ependymal cells during development. An immunocytochemical study". Brain Research 200 (1): 13–21. October 1980. doi:10.1016/0006-8993(80)91090-2. PMID 6998542.
- ↑ "The immunoreactivity of glial fibrillary acidic protein in mesangial cells and podocytes of the glomeruli of rat kidney in vivo and in culture". Biology of the Cell 90 (1): 53–61. January 1998. doi:10.1016/s0248-4900(98)80232-3. PMID 9691426.
- ↑ "Glial fibrillary acidic protein immunoreactivity in adrenocortical and Leydig cells of the Syrian golden hamster (Mesocricetus auratus)". Journal of Neuroimmunology 35 (1–3): 119–129. December 1991. doi:10.1016/0165-5728(91)90167-6. PMID 1720132.
- ↑ "Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules". Acta Histochemica 104 (1): 39–49. 2002. doi:10.1078/0065-1281-00630. PMID 11993850.
- ↑ "Rapid identification of glial cells in human amniotic fluid with indirect immunofluorescence". Acta Cytologica 28 (4): 393–400. 1984. PMID 6205529.
- ↑ "Positivity to glial fibrillary acidic protein in bone, cartilage, and chordoma". Journal of Surgical Oncology 41 (1): 22–26. May 1989. doi:10.1002/jso.2930410109. PMID 2654484.
- ↑ "Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture". Gut 43 (1): 128–133. July 1998. doi:10.1136/gut.43.1.128. PMID 9771417.
- ↑ "Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures". Brain Research 1158: 103–115. July 2007. doi:10.1016/j.brainres.2007.04.070. PMID 17555726.
- ↑ "Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000)". Neurochemical Research 25 (9–10): 1439–1451. October 2000. doi:10.1023/A:1007677003387. PMID 11059815.
- ↑ "Human glial fibrillary acidic protein: complementary DNA cloning, chromosome localization, and messenger RNA expression in human glioma cell lines of various phenotypes". Cancer Research 51 (5): 1553–1560. March 1991. PMID 1847665.
- ↑ 13.0 13.1 "Molecular cloning and primary structure of human glial fibrillary acidic protein". Proceedings of the National Academy of Sciences of the United States of America 86 (13): 5178–5182. July 1989. doi:10.1073/pnas.86.13.5178. PMID 2740350. Bibcode: 1989PNAS...86.5178R.
- ↑ "Sorting out IF networks: consequences of domain swapping on IF recognition and assembly". The Journal of Cell Biology 113 (5): 1111–1124. June 1991. doi:10.1083/jcb.113.5.1111. PMID 1710225.
- ↑ "Molecular interactions in paracrystals of a fragment corresponding to the alpha-helical coiled-coil rod portion of glial fibrillary acidic protein: evidence for an antiparallel packing of molecules and polymorphism related to intermediate filament structure". The Journal of Cell Biology 109 (1): 225–234. July 1989. doi:10.1083/jcb.109.1.225. PMID 2745549.
- ↑ "Intermediate filaments: structure, dynamics, function, and disease". Annual Review of Biochemistry 63: 345–382. 1994. doi:10.1146/annurev.bi.63.070194.002021. PMID 7979242.
- ↑ "In vitro differentiation of cultured human CD34+ cells into astrocytes". Neurology India 61 (4): 383–388. 2013. doi:10.4103/0028-3886.117615. PMID 24005729.
- ↑ "Distribution of five clinically important neuroglial proteins in the human brain". Molecular Brain 15 (1): 52. June 2022. doi:10.1186/s13041-022-00935-6. PMID 35765081.
- ↑ "Regulation of the Glial Fibrillary Acidic Protein (GFAP) and of its Encoding mRNA in the Developing Brain and in Cultured Astrocytes". Molecular Aspects of Development and Aging of the Nervous System. Advances in Experimental Medicine and Biology. 265. 1990. pp. 41–52. doi:10.1007/978-1-4757-5876-4_4. ISBN 978-1-4757-5878-8.
- ↑ "GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination". Neuron 17 (4): 607–615. October 1996. doi:10.1016/S0896-6273(00)80194-4. PMID 8893019.
- ↑ "Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons". The Journal of Cell Biology 112 (6): 1205–1213. March 1991. doi:10.1083/jcb.112.6.1205. PMID 1999469.
- ↑ Online Mendelian Inheritance in Man (OMIM) Glial Fibrillary Acidic Protein, GFAP -137780
- ↑ "Phosphorylation of glial fibrillary acidic protein and vimentin by cytoskeletal-associated intermediate filament protein kinase activity in astrocytes". Journal of Neurochemistry 58 (1): 320–327. January 1992. doi:10.1111/j.1471-4159.1992.tb09313.x. PMID 1727439.
- ↑ "Distribution of glial fibrillary acidic protein in normal and gliotic human retina". Basic and Applied Histochemistry 30 (4): 425–432. 1986. PMID 3548695.
- ↑ "Glial filaments are a major brain fraction in infantile neuronal ceroid-lipofuscinosis". Acta Neuropathologica 65 (3–4): 190–194. 1985. doi:10.1007/bf00686997. PMID 4038838.
- ↑ "Autoimmune glial fibrillary acidic protein astrocytopathy resulting in treatment-refractory flaccid paralysis". Multiple Sclerosis and Related Disorders 39: 101924. January 2020. doi:10.1016/j.msard.2019.101924. PMID 31927153.
- ↑ "A case of GFAP-astroglial autoimmunity presenting with reversible parkinsonism". Multiple Sclerosis and Related Disorders 39: 101900. December 2019. doi:10.1016/j.msard.2019.101900. PMID 31881522.
- ↑ "Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord". The Journal of Biophysical and Biochemical Cytology 10 (1): 67–94. May 1961. doi:10.1083/jcb.10.1.67. PMID 13688845.
- ↑ "Glial fibrillary acidic protein in chronic relapsing experimental allergic encephalomyelitis in SJL/J mice". Journal of Neuroscience Research 18 (1): 203–208. 1987. doi:10.1002/jnr.490180129. PMID 3682026.
- ↑ HealthLink (2007-11-25). "Alexander Disease". Medical College of Wisconsin. http://healthlink.mcw.edu/article/921383447.html.
- ↑ "Alexander disease-associated glial fibrillary acidic protein mutations in mice induce Rosenthal fiber formation and a white matter stress response". The Journal of Neuroscience 26 (43): 11162–11173. October 2006. doi:10.1523/JNEUROSCI.3260-06.2006. PMID 17065456.
- ↑ "Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease". Nature Genetics 27 (1): 117–120. January 2001. doi:10.1038/83679. PMID 11138011.
- ↑ "Alexander disease causing mutations in the C-terminal domain of GFAP are deleterious both to assembly and network formation with the potential to both activate caspase 3 and decrease cell viability". Experimental Cell Research 317 (16): 2252–2266. October 2011. doi:10.1016/j.yexcr.2011.06.017. PMID 21756903.
- ↑ 34.0 34.1 "Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. The Stanley Neuropathology Consortium". Molecular Psychiatry 5 (2): 142–149. March 2000. doi:10.1038/sj.mp.4000696. PMID 10822341.
- ↑ "Chronic alcoholics have substantial glial pathology in the forebrain and diencephalon". Alcohol and Alcoholism 2: 253–257. 1994. PMID 8974344.
- ↑ "Human immunodeficiency virus coat protein gp120 inhibits the beta-adrenergic regulation of astroglial and microglial functions". Proceedings of the National Academy of Sciences of the United States of America 90 (4): 1541–1545. February 1993. doi:10.1073/pnas.90.4.1541. PMID 8381971. Bibcode: 1993PNAS...90.1541L.
- ↑ "Down-regulation of glial fibrillary acidic protein expression during acute lytic varicella-zoster virus infection of cultured human astrocytes". Virology 205 (2): 558–562. December 1994. doi:10.1006/viro.1994.1679. PMID 7975257. https://zenodo.org/record/1229976.
- ↑ "Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus". The Journal of Neuroscience 13 (2): 685–702. February 1993. doi:10.1523/JNEUROSCI.13-02-00685.1993. PMID 8381171.
- ↑ 39.0 39.1 "Blood GFAP as an emerging biomarker in brain and spinal cord disorders". Nature Reviews. Neurology 18 (3): 158–172. March 2022. doi:10.1038/s41582-021-00616-3. PMID 35115728.
- ↑ "Protein Found to Predict Brain Injury in Children on ECMO Life Support". Johns Hopkins Children's Center. 19 November 2010. http://www.hopkinschildrens.org/Protein-Found-to-Predict-Brain-Injury-in-Children-on-ECMO-Life-Support.aspx.
- ↑ "Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity". Experimental Cell Research 278 (2): 175–183. August 2002. doi:10.1006/excr.2002.5575. PMID 12169273.
- ↑ "A new splice variant of glial fibrillary acidic protein, GFAP epsilon, interacts with the presenilin proteins". The Journal of Biological Chemistry 277 (33): 29983–29991. August 2002. doi:10.1074/jbc.M112121200. PMID 12058025.
- ↑ "GFAP in health and disease". Progress in Neurobiology 93 (3): 421–443. March 2011. doi:10.1016/j.pneurobio.2011.01.005. PMID 21219963.
Further reading
- "Early mitochondrial dysfunction in an infant with Alexander disease". Pediatric Neurology 35 (4): 293–296. October 2006. doi:10.1016/j.pediatrneurol.2006.03.010. PMID 16996408.
External links
- GeneReviews/NCBI/NIH/UW entry on Alexander disease
- OMIM entries on Alexander disease
- Glial Fibrillary Acidic Protein at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P14136 (Glial fibrillary acidic protein) at the PDBe-KB.
 |

