Biology:Elastin

Elastin is a protein that in humans is encoded by the ELN gene. Elastin is a key component of the extracellular matrix in gnathostomes (jawed vertebrates).[1] It is highly elastic and present in connective tissue allowing many tissues in the body to resume their shape after stretching or contracting.[2] Elastin helps skin to return to its original position when it is poked or pinched. Elastin is also an important load-bearing tissue in the bodies of vertebrates and used in places where mechanical energy is required to be stored.[3]
Function
The ELN gene encodes a protein that is one of the two components of elastic fibers. The encoded protein is rich in hydrophobic amino acids such as glycine and proline, which form mobile hydrophobic regions bounded by crosslinks between lysine residues.[4] Multiple transcript variants encoding different isoforms have been found for this gene.[4] Elastin's soluble precursor is tropoelastin.[5] The characterization of disorder is consistent with an entropy-driven mechanism of elastic recoil. It is concluded that conformational disorder is a constitutive feature of elastin structure and function.[6]
Clinical significance
Deletions and mutations in this gene are associated with supravalvular aortic stenosis (SVAS) and the autosomal dominant cutis laxa.[4] Other associated defects in elastin include Marfan syndrome, emphysema caused by α1-antitrypsin deficiency, atherosclerosis, Buschke-Ollendorff syndrome, Menkes syndrome, pseudoxanthoma elasticum, and Williams syndrome.[7]
Elastosis
Elastosis is the buildup of elastin in tissues, and is a form of degenerative disease.[8] There are a multitude of causes, but the most commons cause is actinic elastosis of the skin, also known as solar elastosis, which is caused by prolonged and excessive sun exposure, a process known as photoaging. Uncommon causes of skin elastosis include elastosis perforans serpiginosa, perforating calcific elastosis and linear focal elastosis.[8]
| Condition | Distinctive features | Histopathology |
|---|---|---|
| Actinic elastosis (most common, also called solar elastosis) |
Elastin replacing collagen fibers of the papillary dermis and reticular dermis | 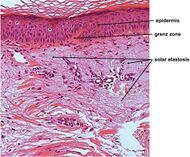
|
| Elastosis perforans serpiginosa | Degenerated elastic fibers and transepidermal perforating canals (arrow in image points at one of them)[9] | 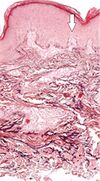
|
| Perforating calcific elastosis | Clumping of short elastic fibers in the dermis.[9] | 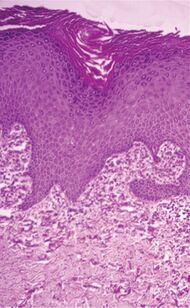
|
| Linear focal elastosis | Accumulation of fragmented elastotic material within the papillary dermis and transcutaneous elimination of elastotic fibers.[9] | 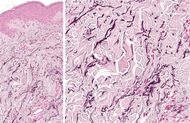
|
Composition

In the body, elastin is usually associated with other proteins in connective tissues. Elastic fiber in the body is a mixture of amorphous elastin and fibrous fibrillin. Both components are primarily made of smaller amino acids such as glycine, valine, alanine, and proline.[7][10] The total elastin ranges from 58 to 75% of the weight of the dry defatted artery in normal canine arteries.[11] Comparison between fresh and digested tissues shows that, at 35% strain, a minimum of 48% of the arterial load is carried by elastin, and a minimum of 43% of the change in stiffness of arterial tissue is due to the change in elastin stiffness.[12]
Tissue distribution
Elastin serves an important function in arteries as a medium for pressure wave propagation to help blood flow and is particularly abundant in large elastic blood vessels such as the aorta. Elastin is also very important in the lungs, elastic ligaments, elastic cartilage, the skin, and the bladder. It is present in jawed vertebrates.[13]
Characteristics
Elastin is a very long-lived protein, with a half-life of over 78 years in humans.[14]
Clinical research
The feasibility of using recombinant human tropoelastin to enable elastin fiber production to improve skin flexibility in wounds and scarring has been studied.[15][16] After subcutaneous injections of recombinant human tropoelastin into fresh wounds it was found there was no improvement in scarring or the flexibility of the eventual scarring.[15][16]
Biosynthesis
Tropoelastin precursors
Elastin is made by linking together many small soluble precursor tropoelastin protein molecules (50-70 kDa), to make the final massive, insoluble, durable complex. The unlinked tropoelastin molecules are not normally available in the cell, since they become crosslinked into elastin fibres immediately after their synthesis by the cell and export into the extracellular matrix.[17]
Each tropoelastin consists of a string of 36 small domains, each weighing about 2 kDa in a random coil conformation. The protein consists of alternating hydrophobic and hydrophilic domains, which are encoded by separate exons, so that the domain structure of tropoelastin reflects the exon organization of the gene. The hydrophilic domains contain Lys-Ala (KA) and Lys-Pro (KP) motifs that are involved in crosslinking during the formation of mature elastin. In the KA domains, lysine residues occur as pairs or triplets separated by two or three alanine residues (e.g. AAAKAAKAA) whereas in KP domains the lysine residues are separated mainly by proline residues (e.g. KPLKP).
Aggregation
Tropoelastin aggregates at physiological temperature due to interactions between hydrophobic domains in a process called coacervation. This process is reversible and thermodynamically controlled and does not require protein cleavage. The coacervate is made insoluble by irreversible crosslinking.
Crosslinking
To make mature elastin fibres, the tropoelastin molecules are cross-linked via their lysine residues with desmosine and isodesmosine cross-linking molecules. The enzyme that performs the crosslinking is lysyl oxidase, using an in vivo Chichibabin pyridine synthesis reaction.[18]
Molecular biology
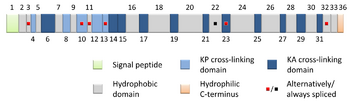
In mammals, the genome only contains one gene for tropoelastin, called ELN. The human ELN gene is a 45 kb segment on chromosome 7, and has 34 exons interrupted by almost 700 introns, with the first exon being a signal peptide assigning its extracellular localization. The large number of introns suggests that genetic recombination may contribute to the instability of the gene, leading to diseases such as SVAS. The expression of tropoelastin mRNA is highly regulated under at least eight different transcription start sites.
Tissue specific variants of elastin are produced by alternative splicing of the tropoelastin gene. There are at least 11 known human tropoelastin isoforms. these isoforms are under developmental regulation, however there are minimal differences among tissues at the same developmental stage.[7]
See also
- Cutis laxa
- Elastic fibers
- Elastin receptor
- Resilin: an invertebrate protein
- Williams syndrome
References
- ↑ "Elastin". Advances in Protein Chemistry 70: 437–461. 2005. doi:10.1016/S0065-3233(05)70013-9. ISBN 9780120342709. PMID 15837523.
- ↑ "Elastin architecture". Matrix Biology 84: 4–16. November 2019. doi:10.1016/j.matbio.2019.07.005. PMID 31301399.
- ↑ "The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis". Cell 73 (1): 159–168. April 1993. doi:10.1016/0092-8674(93)90168-P. PMID 8096434.
- ↑ 4.0 4.1 4.2 "Entrez Gene: elastin". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=2006.
- ↑ "Elastin (ELN)". http://wiki.medpedia.com/Elastin_%28ELN%29.
- ↑ "Structural disorder and dynamics of elastin". Biochemistry and Cell Biology 88 (2): 239–250. April 2010. doi:10.1139/o09-161. PMID 20453927.
- ↑ 7.0 7.1 7.2 "Biochemistry of tropoelastin". European Journal of Biochemistry 258 (1): 1–18. November 1998. doi:10.1046/j.1432-1327.1998.2580001.x. PMID 9851686.
- ↑ 8.0 8.1 "Elastosis". https://dermnetnz.org/topics/elastosis/.
- ↑ 9.0 9.1 9.2 "Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues". Scientifica 2012: 598262. 2012. doi:10.6064/2012/598262. PMID 24278718.
-Creative Commons Attribution 3.0 Unported license - ↑ "Elastic fibres". Journal of Cell Science 115 (Pt 14): 2817–2828. July 2002. doi:10.1242/jcs.115.14.2817. PMID 12082143.
- ↑ "Collagen and elastin content in canine arteries selected from functionally different vascular beds". Circulation Research 19 (2): 394–399. August 1966. doi:10.1161/01.res.19.2.394. PMID 5914851.
- ↑ "Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves". American Journal of Physiology. Heart and Circulatory Physiology 295 (4): H1451–H1459. October 2008. doi:10.1152/ajpheart.00127.2008. PMID 18660454.
- ↑ "Evolution of Elastin Structure". Elastin and Elastic Tissue. Advances in Experimental Medicine and Biology. 79. 1977. pp. 291–312. doi:10.1007/978-1-4684-9093-0_27. ISBN 978-1-4684-9095-4.
- ↑ "Protein homeostasis: live long, won't prosper". Nature Reviews. Molecular Cell Biology 14 (1): 55–61. January 2013. doi:10.1038/nrm3496. PMID 23258296.
- ↑ 15.0 15.1 "New Nanotechnologies for the Treatment and Repair of Skin Burns Infections". International Journal of Molecular Sciences 21 (2): 393. January 2020. doi:10.3390/ijms21020393. PMID 31936277.
- ↑ 16.0 16.1 "Treatment of Burn and Surgical Wounds With Recombinant Human Tropoelastin Produces New Elastin Fibers in Scars". Journal of Burn Care & Research 38 (5): e859–e867. 1 September 2017. doi:10.1097/BCR.0000000000000507. PMID 28221299.
- ↑ "Extracellular Assembly of the Elastin Cable Line Element in the Developing Lung". Anatomical Record 300 (9): 1670–1679. September 2017. doi:10.1002/ar.23603. PMID 28380679.
- ↑ "Two new elastin cross-links having pyridine skeleton. Implication of ammonia in elastin cross-linking in vivo". The Journal of Biological Chemistry 276 (16): 12579–12587. April 2001. doi:10.1074/jbc.M009744200. PMID 11278561.
Further reading
- "Elastin gene study of infants with isolated congenital ductus arteriosus aneurysm". Acta Cardiologica 64 (3): 363–369. June 2009. doi:10.2143/ac.64.3.2038023. PMID 19593948.
- "Elastin as a self-organizing biomaterial: use of recombinantly expressed human elastin polypeptides as a model for investigations of structure and self-assembly of elastin". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 357 (1418): 185–189. February 2002. doi:10.1098/rstb.2001.1027. PMID 11911775.
- "Differential regulation of elastic fiber formation by fibulin-4 and -5". The Journal of Biological Chemistry 284 (36): 24553–24567. September 2009. doi:10.1074/jbc.M109.019364. PMID 19570982.
- "Functional consequences of homocysteinylation of the elastic fiber proteins fibrillin-1 and tropoelastin". The Journal of Biological Chemistry 285 (2): 1188–1198. January 2010. doi:10.1074/jbc.M109.021246. PMID 19889633.
- "Comparison between human fetal and adult skin". Archives of Dermatological Research 302 (1): 47–55. January 2010. doi:10.1007/s00403-009-0989-8. PMID 19701759.
- "Integrative predictive model of coronary artery calcification in atherosclerosis". Circulation 120 (24): 2448–2454. December 2009. doi:10.1161/CIRCULATIONAHA.109.865501. PMID 19948975.
- "Association of genetic variants with chronic kidney disease in individuals with different lipid profiles". International Journal of Molecular Medicine 24 (2): 233–246. August 2009. doi:10.3892/ijmm_00000226. PMID 19578796.
- "Soluble elastin decreases in the progress of atheroma formation in human aorta". Circulation Journal 73 (11): 2154–2162. November 2009. doi:10.1253/circj.cj-09-0104. PMID 19755752.
- "Fibulin-4 regulates expression of the tropoelastin gene and consequent elastic-fibre formation by human fibroblasts". The Biochemical Journal 423 (1): 79–89. September 2009. doi:10.1042/BJ20090993. PMID 19627254.
- "Human tropoelastin sequence: dynamics of polypeptide coded by exon 6 in solution". Biopolymers 91 (11): 943–952. November 2009. doi:10.1002/bip.21282. PMID 19603496. https://oatao.univ-toulouse.fr/8663/8/Tintar_8663.pdf.
- "Homology models for domains 21-23 of human tropoelastin shed light on lysine crosslinking". Biochemical and Biophysical Research Communications 396 (4): 870–873. June 2010. doi:10.1016/j.bbrc.2010.05.013. PMID 20457133.
- "Identification of fetal and maternal single nucleotide polymorphisms in candidate genes that predispose to spontaneous preterm labor with intact membranes". American Journal of Obstetrics and Gynecology 202 (5): 431.e1–431.34. May 2010. doi:10.1016/j.ajog.2010.03.026. PMID 20452482.
- "Lack of association of polymorphisms in elastin with pseudoexfoliation syndrome and glaucoma". Journal of Glaucoma 19 (7): 432–436. September 2010. doi:10.1097/IJG.0b013e3181c4b0fe. PMID 20051886.
- "Cellular senescence of human mammary epithelial cells (HMEC) is associated with an altered MMP-7/HB-EGF signaling and increased formation of elastin-like structures". Mechanisms of Ageing and Development 130 (10): 657–669. October 2009. doi:10.1016/j.mad.2009.08.001. PMID 19682489.
- "Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease". Gastroenterology 139 (1): 130–9.e24. July 2010. doi:10.1053/j.gastro.2010.03.044. PMID 20346360.
- "Elastin: relation of protein and gene structure to disease". Laboratory Investigation; A Journal of Technical Methods and Pathology 51 (6): 605–623. December 1984. PMID 6150137.
- "Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3". The Journal of Biological Chemistry 284 (42): 28616–28623. October 2009. doi:10.1074/jbc.M109.017525. PMID 19617625.
- "A novel elastin gene mutation resulting in an autosomal dominant form of cutis laxa". Archives of Dermatology 140 (9): 1135–1139. September 2004. doi:10.1001/archderm.140.9.1135. PMID 15381555.
- "Identification and characterization of seven novel mutations of elastin gene in a cohort of patients affected by supravalvular aortic stenosis". European Journal of Human Genetics 18 (3): 317–323. March 2010. doi:10.1038/ejhg.2009.181. PMID 19844261.
- "The role of collagen and elastin in aged skin: an image processing approach". Micron 35 (3): 173–177. 2004. doi:10.1016/j.micron.2003.11.003. PMID 15036271.
External links
- Elastin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Histology image: 21402loa – Histology Learning System at Boston University
- GeneReviews/NIH/NCBI/UW entry on Williams or Williams-Beuren Syndrome
- The Elastin Protein
- Microfibril
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |
