Biology:Fibrillin
| fibrillin 1 | |
|---|---|
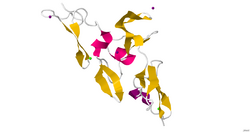 Crystallographic structure of the cbEGF9-hybrid2-cbEGF10 region of human fibrillin 1.[1] | |
| Identifiers | |
| Symbol | FBN1 |
| Alt. symbols | FBN, MFS1, WMS |
| NCBI gene | 2200 |
| HGNC | 3603 |
| OMIM | 134797 |
| PDB | 2W86 |
| RefSeq | NM_000138 |
| UniProt | P35555 |
| Other data | |
| Locus | Chr. 15 q21.1 |
| fibrillin 2 | |
|---|---|
| Identifiers | |
| Symbol | FBN2 |
| Alt. symbols | CCA |
| NCBI gene | 2201 |
| HGNC | 3604 |
| OMIM | 121050 |
| RefSeq | NM_001999 |
| UniProt | P35556 |
| Other data | |
| Locus | Chr. 5 q23-q31 |
| fibrillin 3 | |
|---|---|
| Identifiers | |
| Symbol | FBN3 |
| NCBI gene | 84467 |
| HGNC | 18794 |
| OMIM | 608529 |
| RefSeq | NM_032447 |
| UniProt | Q75N90 |
| Other data | |
| Locus | Chr. 19 p13 |
Fibrillin is a glycoprotein, which is essential for the formation of elastic fibers found in connective tissue.[2] Fibrillin is secreted into the extracellular matrix by fibroblasts and becomes incorporated into the insoluble microfibrils, which appear to provide a scaffold for deposition of elastin.[3]
Clinical aspects
Marfan syndrome is a genetic disorder of the connective tissue caused by defected FBN1 gene. Mutations in FBN1 and FBN2 are also sometimes associated with adolescent idiopathic scoliosis.[4]
Types
Fibrillin-1
Fibrillin-1 is a major component of the microfibrils that form a sheath surrounding the amorphous elastin. It is believed that the microfibrils are composed of end-to-end polymers of fibrillin. To date, 3 forms of fibrillin have been described. The fibrillin-1 protein was isolated by Engvall in 1986,[5] and mutations in the FBN1 gene cause Marfan syndrome.[6][7]
This protein is found in humans, and its gene is found on chromosome 15. At present more than 1500 different mutations have been described.[1][7]
Structure
There is no complete, high-resolution structure of fibrillin-1. Instead, short fragments have been produced recombinantly and their structures solved by X-ray crystallography or using NMR spectroscopy. A recent example is the structure of the fibrillin-1 hybrid2 domain, in context of its flanking calcium binding epidermal growth factor domains, which was determined using X-ray crystallography to a resolution of 1.8 Å.[1] The microfibrils that are made up of fibrillin protein are responsible for different cell-matrix interactions in the human body.
Fibrillin-2
Fibrillin-2 was isolated in 1994 by Zhang[8] and is thought to play a role in early elastogenesis. Mutations in the fibrillin-2 gene have been linked to Beals syndrome.
Fibrillin-3
More recently, fibrillin-3 was described and is believed to be located mainly in the brain.[9] Along with the brain, fibrillin-3 has been localized in the gonads and ovaries of field mice.
Fibrillin-4
Fibrillin-4 was first discovered in zebrafish, and has a sequence similar to fibrillin-2.[10]
References
- ↑ 1.0 1.1 1.2 PDB: 2W86; "Structure and interdomain interactions of a hybrid domain: a disulphide-rich module of the fibrillin/LTBP superfamily of matrix proteins". Structure 17 (5): 759–68. May 2009. doi:10.1016/j.str.2009.03.014. PMID 19446531.
- ↑ "Fibrillin: from microfibril assembly to biomechanical function". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 357 (1418): 207–17. February 2002. doi:10.1098/rstb.2001.1029. PMID 11911778.
- ↑ Singh (2006). Textbook of human histology. Jaypee Brothers Publishers. pp. 64–. ISBN 978-81-8061-809-3. https://books.google.com/books?id=Ej22iANgNkoC&pg=PA64. Retrieved 9 December 2010.
- ↑ "Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis". Human Molecular Genetics 23 (19): 5271–82. October 2014. doi:10.1093/hmg/ddu224. PMID 24833718.
- ↑ "Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils". The Journal of Cell Biology 103 (6 Pt 1): 2499–509. December 1986. doi:10.1083/jcb.103.6.2499. PMID 3536967.
- ↑ "New therapeutic approaches to mendelian disorders". The New England Journal of Medicine 363 (9): 852–63. August 2010. doi:10.1056/NEJMra0907180. PMID 20818846.
- ↑ 7.0 7.1 "Perspectives on the revised Ghent criteria for the diagnosis of Marfan syndrome". The Application of Clinical Genetics 8: 137–55. 2015. doi:10.2147/TACG.S60472. PMID 26124674.
- ↑ "Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices". The Journal of Cell Biology 124 (5): 855–63. March 1994. doi:10.1083/jcb.124.5.855. PMID 8120105.
- ↑ "Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues". Genomics 83 (3): 461–72. March 2004. doi:10.1016/j.ygeno.2003.08.023. PMID 14962672.
- ↑ "Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis". Developmental Dynamics 237 (10): 2844–61. October 2008. doi:10.1002/dvdy.21705. PMID 18816837.
External links
- Fibrillin at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
