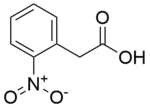
Chemistry:(2-Nitrophenyl)acetic acid
| |||
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-(2-nitrophenyl)acetic acid [2]
| |||
| Other names
Benzeneacetic acid, 2-nitro-[1]
o-Nitrophenylacetic acid 2-nitrophenylacetic acid (ortho-Nitrophenyl)acetic acid acetic acid, (o-nitrophenyl) 2-(o-nitrophenyl)acetic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C8H7NO4 | |||
| Molar mass | 181.15 g/mol | ||
| Appearance | Yellow to Pale Brown Crystalline Powder | ||
| Density | 1.4 g/cm3[3] | ||
| Boiling point | 141 °C (286 °F; 414 K) | ||
| 0.1417% (20 °C)[4] | |||
| Hazards | |||
| Main hazards | Irritant | ||
| Safety data sheet | MSDS | ||
| GHS pictograms |  
| ||
| GHS Signal word | Warning | ||
| H315, H319, H335, H341 | |||
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P317Script error: No such module "Preview warning".Category:GHS errors, P332, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related derivatives;
related aromatic compounds |
phenylacetic acid,4-nitrophenylacetic acid; 4-nitrophenol, 2-nitrodiphenylamine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2-Nitrophenylacetic acid is an organic compound used in organic synthesis that has also been used as an herbicide. It is a derivative of phenylacetic acid, containing a phenyl functional group, a carboxylic acid functional group, and a nitro functional group. It is an important reagent for many organic reactions, especially for the formation of heterocycles.
Synthesis
This compound may be prepared by the nitration of phenylacetic acid.[5]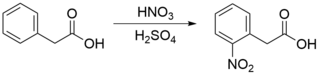
Applications
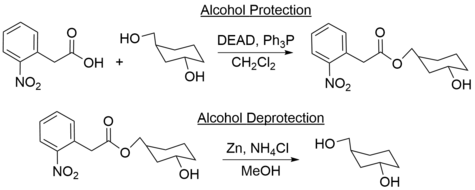
In organic synthesis, 2-nitrophenylacetic acid can be used as a protecting group for primary alcohols. The alcohol is esterified with 2-nitrophenylacetic acid, proceeding through the acid chloride or acid anhydride. The acid itself can also protect the alcohol through the Mitsunobu reaction: reacting the alcohol and the acid with diethyl azidocarboxylate and triphenylphosphine in dichloromethane. The protecting group is selectively removed using zinc and ammonium chloride, and is compatible with other existing alcohol protecting groups.[6]
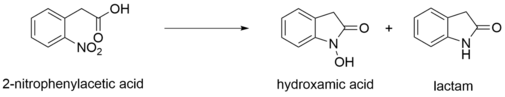
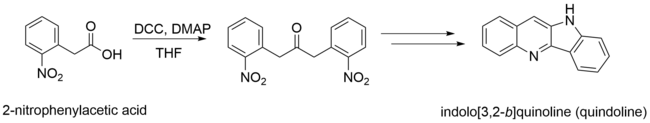
In addition, 2-nitrophenylacetic acid is a precursor for many heterocycles. Complete reduction of 2-nitrophenylacetic acid yields anilines, which quickly cyclize to form lactams.[7][8] Partial reductive cyclization of the acids using weaker reducing agents forms hydroxamic acids.[8]
Both of these processes are useful in the synthesis of many biologically active molecules. 2-nitrophenylacetic acid is a precursor of quindoline, which although it does not have many practical applications on its own, quindoline derivatives and modifications can be treated as enzyme inhibitors and anticancer agents.[9]
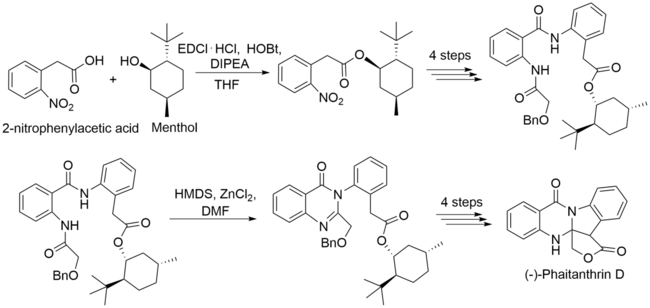
Derivatives of 2-nitrophenylacetic acids are useful in total synthesis for their ability to form heterocycles. 2-nitrophenylacetic acid is a precursor to (−)-phaitanthrin D, a clinically useful molecule originally isolated from the Phaius mishmensis orchid.[10] The carboxylic acid on the 2-nitrophenylacetic acid is first protected using menthol, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCl), hydroxybenzotriazole(HOBt) and N,N-iisopropylethylamine(DIPEA). A pattern of reducing the nitro group to an amino group and subsequently forming amides by the addition to carboxylic acids (namely nitrobenzoic acid) occurs. Reductive cyclization of the subsequent product using hexamethyldisilazane, zinc chloride and dimethylformamide forms the disubstituted heterocycle present in the (−)-phaitantrin D molecule.
Outside of organic synthesis, 2-nitrophenylacetic acid has been used as an herbicide, as it displays selective herbicidal properties.[11] It has also been used as an internal standard for measurement of salicylamide-O-acetic acid (an anti-asthma drug) using high performance liquid chromatography. [12]
References
- ↑ "Benzeneacetic acid, 2-nitro-". https://webbook.nist.gov/cgi/cbook.cgi?ID=3740-52-1.
- ↑ "2-Nitrophenylacetic acid (compound)". National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/77337#section=Names-and-Identifiers.
- ↑ "AJ1130000". https://www.chemspider.com/Chemical-Structure.69754.html?rid=3a5361a1-eefa-4909-9499-b988e7838fcd#suppInfoTab.
- ↑ Zhou, Yanyan; Wu, Jiaxin; Wang, Jian; Zhao, Hongkun (13 August 2020). "Equilibrium Solubility and Dissolution Property Analysis of 2-Nitrophenylacetic Acid in 13 Pure Solvents at Elevated Temperatures". Journal of Chemical & Engineering Data 65 (8): 4157–4165. doi:10.1021/acs.jced.0c00543.
- ↑ Sohail, Muhammad; Raza, Abdul Rauf (February 2012). "A Novel One Pot Synthesis of o-Nitrophenylacetic Acid and Unexpected p-Nitrobenzoic Acid by HNO3-Mediated CH2 Extrusion Reaction of Phenylacetic Acid". Chinese Journal of Chemistry 30 (2): 353–356. doi:10.1002/cjoc.201180458.
- ↑ Daragics, Katalin; Fügedi, Péter (7 May 2010). "(2-Nitrophenyl)acetyl: A New, Selectively Removable Hydroxyl Protecting Group". Organic Letters 12 (9): 2076–2079. doi:10.1021/ol100562f. PMID 20361745.
- ↑ Wright, William B.; Collins, Kenneth H. (January 1956). "Cyclic Hydroxamic Acids Derived from Indole" (in en). Journal of the American Chemical Society 78 (1): 221–224. doi:10.1021/ja01582a061. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01582a061.
- ↑ 8.0 8.1 Ichire, Ogar; Jans, Petra; Parfenov, Galina; Dounay, Amy B. (2017-02-08). "A safe and selective method for reduction of 2-nitrophenylacetic acid systems to N-aryl hydroxamic acids using continuous flow hydrogenation" (in en). Tetrahedron Letters 58 (6): 582–585. doi:10.1016/j.tetlet.2017.01.008. ISSN 0040-4039. https://www.sciencedirect.com/science/article/pii/S0040403917300084.
- ↑ Singer, Jamie M.; Barr, Bridget M.; Coughenour, Linda L.; Gregory, Tracy F.; Walters, Michael A. (2005-10-15). "8-Substituted 3,4-dihydroquinolinones as a novel scaffold for atypical antipsychotic activity" (in en). Bioorganic & Medicinal Chemistry Letters 15 (20): 4560–4563. doi:10.1016/j.bmcl.2005.06.097. ISSN 0960-894X. PMID 16087333. https://www.sciencedirect.com/science/article/pii/S0960894X0500867X.
- ↑ Vaidya, Sagar; Argade, Narshinha (2016-05-17). "Synthesis of (–)-Phaitanthrin D and (+)-Dihydropyrroloindoloquinazolinone" (in en). Synthesis 48 (17): 2896–2903. doi:10.1055/s-0035-1562098. ISSN 0039-7881. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0035-1562098.
- ↑ Back, Gayle E.; Dahle, Norman A. (July 6, 1971). "NITROPHENYLACETIC ACID DERVATIVES". https://patentimages.storage.googleapis.com/93/fb/dc/5c755cf73a191f/US3591623.pdf.
- ↑ Schulz, H. -U.; Kraas, E. (1985-01-01). "Determination of the theophylline solubilizer salicylamide-O-acetic acid in serum and urine using high-performance liquid chromatography" (in en). Journal of Pharmaceutical and Biomedical Analysis 3 (5): 469–475. doi:10.1016/0731-7085(85)80062-5. ISSN 0731-7085. PMID 16867660. https://dx.doi.org/10.1016/0731-7085%2885%2980062-5.
 |