Chemistry:1,4-Oxathiane
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,4-Oxathiane | |
| Other names
1,4-Thioxane; p-Thioxane; 1-Oxa-4-thiacyclohexane
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8OS | |
| Molar mass | 104.17 g·mol−1 |
| Melting point | −17 °C (1 °F; 256 K) |
| Boiling point | 147.0 °C (296.6 °F; 420.1 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2830 mg/kg oral rat |
| Related compounds | |
Related compounds
|
1,4-dioxane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,4-Oxathiane is a heterocyclic compound containing one oxygen atom and one sulfur atom at opposite corners of a saturated six-membered ring. By systematic numbering, the oxygen atom is position number 1, sulfur is number 4, and positions 2, 3, 5, and 6 are carbon atoms.
Production
1,4-Oxathiane can be produced from low cost ingredients by heating ethylene glycol or ethylene oxide with hydrogen sulfide. Alternate ways are to dehydrate bis(hydroxy ethyl) sulfide by heating with potassium hydrogen sulfate. These reactions also form 1,4-dithiane as a byproduct.[1]
The original 1912 preparation of 1,4-oxathiane involved iodoethyl ether with potassium sulfide in alcohol. A similar method used 2-chloroethyl ether.[1]
Reactions
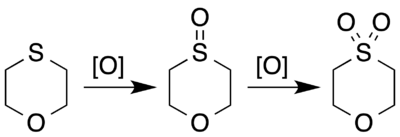
The sulfur atom in 1,4-oxathiane can undergo reaction as other substituted sulfides can. It can be oxidised to a sulfoxide with calcium hypochlorite or sodium periodate,[2] or continuing to a sulfone.
It can react with ammonia to form diimines.[3]
With elemental chlorine below 0 °C, 3-chloro-1,4-oxathiane is formed. Above 0 °C, 2,3-dichloro-1,4-oxathiane results and further chlorination yields 2,3,5,6-tetrachloro-1,4-oxathiane.[1]
With hydrofluoric acid, 1,4-oxathiane is undergoes electrophilic fluorination to yield perfluoro-1,4-oxathiane: all eight hydrogen atoms are replaced with fluorine substituents, and also four fluorine atoms are attached to the sulfur atom.[1]
With elemental bromine in ether, an oxathianium salt is formed. In this an extra bromine atom bonds to the sulfur atom which gets a positive charge. To balance this, a bromide ion forms to make up a salt. Similarly iodine in acetic acid reacts to make 4-iodo-1,4-oxathianium iodide. Heating 1,4-oxathiane with ethyl iodide yields 4-ethyl-1,4-oxathianium iodide.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Breslow, David S.; Skolnik, Herman (2009) (in en). Multi-Sulfur and Sulfur and Oxygen Five- and Six-Membered Heterocycles. John Wiley & Sons. pp. 823–828. ISBN 978-0-470-18833-0. https://books.google.com/books?id=CgRLJvZYBpYC&pg=PA828.
- ↑ Yamamoto, ed (2004). "Product Subclass 8: Aluminum Oxide (Alumina)". Category 1, Organometallics. doi:10.1055/sos-SD-007-00192. ISBN 9783131122117.
- ↑ Kambe, ed (2008). "Product Subclass 2: Cyclic Dialkyl Sulfones and Derivatives". Category 5, Compounds with One Saturated Carbon Heteroatom Bond. doi:10.1055/sos-SD-039-00922. ISBN 9783131189219.
 |