Chemistry:Calcium hypochlorite
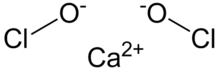
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1748 2208 |
| |
| |
| Properties | |
| Ca(OCl) 2 | |
| Molar mass | 142.98 g·mol−1 |
| Appearance | white/gray powder |
| Density | 2.35 g/cm3 (20 °C) |
| Melting point | 100 °C (212 °F; 373 K) |
| Boiling point | 175 °C (347 °F; 448 K) decomposes |
| 21 g/(100 mL) at 25 °C | |
| Solubility | reacts in alcohol |
| Hazards | |
| Safety data sheet | ICSC 0638 |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H272, H302, H314, H400 | |
| P210, P220, P221, P260, P264, P270, P273, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P370+378, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
850 mg/kg (oral, rat) |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium hypochlorite is an inorganic compound with chemical formula Ca(ClO)
2, also written as Ca(OCl)
2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite.[1] "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical.[2] It is the main active ingredient of commercial products called bleaching powder,[lower-alpha 1] used for water treatment and as a bleaching agent.[3]
History
Charles Tennant and Charles Macintosh developed an industrial process in the late 18th century for the manufacture of chloride of lime, patenting it in 1799.[4] Tennant's process is essentially still used today,[4][3] and became of military importance during World War I, because calcium hypochlorite was the active ingredient in trench disinfectant.[4]
Uses
Sanitation
Calcium hypochlorite is commonly used to sanitize public swimming pools and disinfect drinking water. Generally the commercial substances are sold with a purity of 65% to 73% with other chemicals present, such as calcium chloride and calcium carbonate, resulting from the manufacturing process. In solution, calcium hypochlorite could be used as a general purpose sanitizer,[5] but due to calcium residue (making the water harder), sodium hypochlorite (bleach) is usually preferred.
Organic chemistry
Calcium hypochlorite is a general oxidizing agent and therefore finds some use in organic chemistry.[6] For instance the compound is used to cleave glycols, α-hydroxy carboxylic acids and keto acids to yield fragmented aldehydes or carboxylic acids.[7] Calcium hypochlorite can also be used in the haloform reaction to manufacture chloroform.[8] Calcium hypochlorite can be used to oxidize thiol and sulfide byproducts in organic synthesis and thereby reduce their odour and make them safe to dispose of.[9] The reagent used in organic chemistry is similar to the sanitizer at ~70% purity.[10]
Production
Calcium hypochlorite is produced industrially by reaction of moist slaked calcium hydroxide with chlorine gas. The one-step reaction is shown below:[3]
- 2 Cl
2 + 2 Ca(OH)
2 → CaCl
2 + Ca(OCl)
2 + 2 H
2O
Industrial setups allow for the reaction to be conducted in stages to give various compositions, each producing different ratios of calcium hypochlorite, unconverted lime, and calcium chloride.[3] In one process, the chloride-rich first stage water is discarded, while the solid precipitate is dissolved in a mixture of water and lye for another round of chlorination to reach the target purity.[2] Commercial calcium hypochlorite consists of anhydrous Ca(OCl)
2, dibasic calcium hypochlorite Ca
3(OCl)
2(OH)
4 (also written as Ca(OCl)
2 · 2Ca(OH)
2), and dibasic calcium chloride Ca
3Cl
2(OH)
4 (also written as CaCl
2 · 2Ca(OH)
2).[11][12]
Reactions
Calcium hypochlorite reacts rapidly with acids producing calcium chloride, chlorine gas, and water:[citation needed]
- Ca(ClO)
2 + 4 HCl → CaCl
2 + 2 Cl
2 + 2 H
2O
Safety
It is a strong oxidizing agent, as it contains a hypochlorite ion at the valence +1 (redox state: Cl+1).[citation needed]
Calcium hypochlorite should not be stored wet and hot, or near any acid, organic materials, or metals. The unhydrated form is safer to handle.[citation needed]
See also
References
- ↑ also chlorine powder, chloride of lime, chlorinated lime, "dry chlorine"
- ↑ "Key operating strategies for chlorine disinfection operating systems". http://www.environmental-expert.com/Files%5C5306%5Carticles%5C13866%5C500.pdf.
- ↑ 2.0 2.1 "Calcium Hypochlorite - 3V Tech" (in en). https://www.3v-tech.com/en/technologies-and-solutions/13/calcium-hypochlorite.
- ↑ 3.0 3.1 3.2 3.3 Vogt, H.; Balej, J; Bennett, J. E.; Wintzer, P.; Sheikh, S. A.; Gallone, P.; Vasudevan, S.; Pelin, K. (2010). Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a06_483.pub2. ISBN 978-3527306732.
- ↑ 4.0 4.1 4.2 "Calcium hypochlorite". Chemistry World. https://www.chemistryworld.com/podcasts/calcium-hypochlorite/3008985.article.
- ↑ Chemical Products Synopsis: Calcium Hypochlorite (Technical report). Asbuiy Park, NJ: Mannsvile Chemical Products. 1987.
- ↑ Nwaukwa, Stephen; Keehn, Philip (1982). "The oxidation of aldehydes to acids with calcium hypochlorite [Ca(ClO)2]". Tetrahedron Letters 23 (31): 3131–3134. doi:10.1016/S0040-4039(00)88577-9.
- ↑ Nwaukwa, Stephen; Keehn, Philip (1982). "Oxidative cleavage of α-diols, α-diones, α-hydroxy-ketones and α-hydroxy- and α-keto acids with calcium hypochlorite [Ca(ClO)2]". Tetrahedron Letters 23 (31): 3135–3138. doi:10.1016/S0040-4039(00)88578-0.
- ↑ Cohen, Julius (1900). Practical Organic Chemistry for Advanced Students. New York: Macmillan & Co.. p. 63. https://books.google.com/books?id=0xRIAAAAIAAJ.
- ↑ National Research Council (1995). Prudent Practices in the Laboratory: Handling and Disposal of Chemicals. Washington, DC: The National Academies Press. p. 161. doi:10.17226/4911. ISBN 978-0-309-05229-0.
- ↑ "8.41799 Calcium hypochlorite for synthesis". https://www.sigmaaldrich.com/BE/en/product/mm/841799. "Assay (iodometric): 67.0 - 75.0 %"
- ↑ W.L Smith, Inorganic Bleaches, Production of Hypochlorite in Handbook of Detergents,Part F, (2009) Ed. U Zoller and Paul Sosis, CRC Press, ISBN 978-0-8247-0349-3
- ↑ Aleksandrova, M.M.; Dmitriev, G.A.; Avojan, R.L. (1968). "The probable model of the crystal structure of the twobase calcium hypochlorite". Armyanskii Khimicheskii Zhurnal 21: 380-386.
External links
 |

