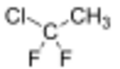
Chemistry:1-Chloro-1,1-difluoroethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-1,1-difluoroethane | |
| Other names
Freon 142b; R-142b; HCFC-142b; Chlorodifluoroethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H3ClF2 | |
| Molar mass | 100.49 g·mol−1 |
| Appearance | Colorless gas[1] |
| Melting point | −130.8 °C (−203.4 °F; 142.3 K)[1] |
| Boiling point | −9.6 °C (14.7 °F; 263.5 K)[1] |
| Slight[1] | |
| Hazards | |
| Main hazards | Asphyxiant |
| 632 °C (1,170 °F; 905 K)[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.[2]
Physiochemical properties
1-Chloro-1,1-difluoroethane is a highly flammable, colorless gas under most atmospheric conditions. It has a boiling point of -10 °C.[1][3] Its critical temperature is near 137 °C.[4]
Applications
HCFC-142b is used as a refrigerant, as a blowing agent for foam plastics production, and as feedstock to make polyvinylidene fluoride (PVDF).[5] It was introduced to replace the chlorofluorocarbons (CFCs) that were initially undergoing a phase-out per the Montreal Protocol, but HCFCs still have a significant ozone-depletion ability. As of year 2020, HCFC's are replaced by non ozone depleting HFCs within many applications.[6]
In the United States, the EPA stated that HCFCs could be used in "processes that result in the transformation or destruction of the HCFCs", such as using HCFC-142b as a feedstock to make PVDF. HCFCs could also be used in equipment that was manufactured before January 1, 2010.[7] The point of these new regulations was to phase-out HCFCs in much the same way that CFCs were phased out. HCFC-142b production in non article 5 countries like the United States was banned on January 1, 2020, under the Montreal Protocol.[6]
Production history
According to the Alternative Fluorocarbons Environmental Acceptability Study (AFEAS), in 2006 global production (excluding India and China who did not report production data) of HCFC-142b was 33,779 metric tons and an increase in production from 2006 to 2007 of 34%.[8]
For the most part, concentrations of HCFCs in the atmosphere match the emission rates that were reported by industries. The exception to this is HCFC-142b which had a higher concentration than the emission rates suggest it should.[9]
Environmental effects


The concentration of HCFC-142b in the atmosphere grew to over 20 parts per trillion by year 2010.[10] It has an ozone depletion potential (ODP) of 0.07.[11] This is low compared to the ODP=1 of trichlorofluoromethane (CFC-11, R-11), which also grew about ten times more abundant in the atmosphere by year 1985 (prior to introduction of HFC-142b and the Montreal Protocol).
HFC-142b is also a minor but potent greenhouse gas. It has an estimated lifetime of about 17 years and a 100-year global warming potential ranging 2300 to 5000.[12][13] This compares to the GWP=1 of carbon dioxide, which had a much greater atmospheric concentration near 400 parts per million in year 2020.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ "Safety Data Sheet for 1-Chloro-1,1-difluoroethane". http://www.refrigerants.com/pdf/SDS%20R142b.pdf.
- ↑ "Addenda d, j, l, m, and t to ANSI/ASHRAE Standard 34-2004". ANSI/ASHRAE Standard 34-2004, Designation and Safety Classification of Refrigerants. Atlanta, GA: American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.. 2007-03-03. http://ashrae.org/File%20Library/docLib/Public/20070613_342004_d_j_l_m_t_only.pdf.
- ↑ Schoen, J. Andrew, "Listing of Refrigerants", Andy's HVAC/R Web Page, http://www.jandrewschoen.com/refchart.pdf, retrieved 2011-12-17
- ↑ "Phaseout of Class II Ozone-Depleting Substances". Environmental Protection Agency. 22 July 2015. https://www.epa.gov/ods-phaseout/phaseout-class-ii-ozone-depleting-substances.
- ↑ 6.0 6.1 "Overview of HCFC Consumption and Available Alternatives For Article 5 Countries". ICF International. 2008. https://ec.europa.eu/clima/sites/clima/files/docs/0007/icf_hcfc_background_doc_en.pdf. Retrieved 2021-02-12.
- ↑ U.S. Government Publishing Office Federal Register 2005 November 4, Protection of Stratospheric Ozone: Notice of Data Availability; Information Concerning the Current and Predicted Use of HCFC-22 and HCFC-142b Pages 67172 - 67174 [FR DOC # 05-22036].
- ↑ "Production and Sales of Fluorocarbons - AFEAS". http://www.afeas.org/overview.php.
- ↑ "Good news from the stratosphere, sort of: Accumulating HCFCs won't stop ozone-hole mending". http://www.ucar.edu/communications/quarterly/spring01/hcfcs.html.
- ↑ 10.0 10.1 "HCFC-142b". NOAA Earth System Research Laboratories/Global Monitoring Division. https://www.esrl.noaa.gov/gmd/hats/gases/HCFC142b.html. Retrieved 2021-02-12.
- ↑ John S. Daniel; Guus J.M. Velders; A.R. Douglass; P.M.D. Forster; D.A. Hauglustaine; I.S.A. Isaksen; L.J.M. Kuijpers; A. McCulloch et al. (2006). "Chapter 8. Halocarbon Scenarios, Ozone Depletion Potentials, and Global Warming Potentials". Scientific Assessment of Ozone Depletion: 2006. Geneva, Switzerland: World Meteorological Organization. http://www.esrl.noaa.gov/csd/assessments/ozone/2006/chapters/chapter8.pdf. Retrieved 9 October 2016.
- ↑ "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731. https://www.ipcc.ch/report/ar5/wg1/.
- ↑ "Refrigerants - Environmental Properties". http://www.engineeringtoolbox.com/Refrigerants-Environment-Properties-d_1220.html. Retrieved 2016-09-12.
 |

