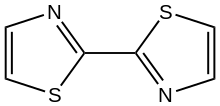
Chemistry:2,2'-Bithiazole
| |
| Identifiers | |
|---|---|
| Properties | |
| C6H4N2S2 | |
| Molar mass | 168.23 g·mol−1 |
| Appearance | white solid |
| Density | 1.82 g/cm3 |
| Melting point | 102.5 °C (216.5 °F; 375.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,2'-Bithiazole is an organic compound with the formula (H
2C
3NS)
2. The molecule consists of two thiazole rings linked by a C-C bond. Diverse isomers are possible depending on the carbon atoms that are coupled, but the 2,2' isomer is common. The compound was first prepared by Ullmann coupling of 2-bromothiazole using copper metal.[1]
2,2'-Bithiazole is planar, according to X-ray crystallography.[2]
Occurrence
thumb| Bleomycin A2, a bithiazole-containing natural product|320px|left 2,2'-Bithiazole itself is mainly of academic interest, but substituted, isomeric bithiazoles have attracted attention in medicinal chemistry. Naturally occurring bithiazoles are derived from cysteine, as are most naturally-occurring thiazoles. Well-studied derivatives are the bleomycins, which feature 2,4'-bithiazole incorporated into glycopeptides. The cystothiazoles are another family of bithiazoles, but they feature 2,5-linkage. Luciferin contains a benzothiazole subunit linked to a thiazolidine (dihydrothiazole) via a 2,2' linkage.[3]
2,2'-Bithiazole forms a variety of coordination complexes.[2]
References
- ↑ Erlenmeyer, H.; Schmid, Erich H. (1939). "Über isostere und strukturähnliche Verbindungen XI. Darstellung und Eigenschaften des 2,2′-Dithiazolyl". Helvetica Chimica Acta 22: 698–700. doi:10.1002/hlca.19390220186.
- ↑ 2.0 2.1 Craig, DC; Goodwin, H. A.; Onggo, D.; Rae, AD (1988). "Coordination of 2,2'-Bithiazole. Spectral, Magnetic and Structural Studies of the Iron(II) and Nickel(II) Complexes". Australian Journal of Chemistry 41 (11): 1625. doi:10.1071/CH9881625.
- ↑ Jin, Zhong (2011). "Muscarine, imidazole, oxazole, and thiazole alkaloids". Natural Product Reports 28 (6): 1143–1191. doi:10.1039/C0NP00074D. PMID 21472175.
 |

