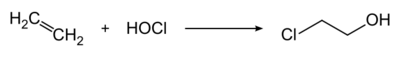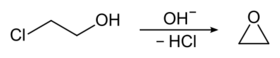Chemistry:2-Chloroethanol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chloroethan-1-ol[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 878139 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 25389 | |||
| KEGG | |||
| MeSH | Ethylene+Chlorohydrin | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1135 | ||
| |||
| |||
| Properties | |||
| C2H5ClO | |||
| Molar mass | 80.51 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | ether-like | ||
| Density | 1.201 g/mL | ||
| Melting point | −62.60 °C; −80.68 °F; 210.55 K | ||
| Boiling point | 127–131 °C; 260–268 °F; 400–404 K | ||
| Miscible[3] | |||
| log P | −0.107 | ||
| Vapor pressure | 700 Pa (at 20 °C) | ||
Refractive index (nD)
|
1.441 | ||
| Thermochemistry | |||
Std enthalpy of
combustion (ΔcH⦵298) |
−1.1914 MJ/mol | ||
| Hazards | |||
| Main hazards | Highly toxic and flammable | ||
| GHS pictograms |  
| ||
| GHS Signal word | DANGER | ||
| H226, H300+310+330Script error: No such module "Preview warning".Category:GHS errors | |||
| P260, P280, P284, P301+310, P302+350 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 55 °C (131 °F; 328 K) | ||
| 425 °C (797 °F; 698 K) | |||
| Explosive limits | 5–16% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LC50 (median concentration)
|
|||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (16 mg/m3) [skin][3] | ||
REL (Recommended)
|
C 1 ppm (3 mg/m3) [skin][3] | ||
IDLH (Immediate danger)
|
7 ppm[3] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the simplest beta-halohydrin (chlorohydrin).[6] This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group.[7]
Synthesis and applications
2-Chloroethanol is produced by treating ethylene with hypochlorous acid:[7]
2-Chloroethanol was once produced on a large scale as a precursor to ethylene oxide:
- HOCH2CH2Cl + NaOH → C2H4O + NaCl + H2O
This application has been supplanted by the more economic direct oxidation of ethylene. Otherwise chloroethanol is still used in the production of pharmaceuticals, biocides, and plasticizers.[7] Many of these applications entail its use in installing 2-hydroxyethyl groups.[8] Several dyes are prepared by the alkylation of aniline derivatives with chloroethanol.[9] It is also used for manufacture of thiodiglycol.
It is a solvent for cellulose acetate and ethyl cellulose, textile printing dyes, in dewaxing, refining of rosin, extraction of pine lignin, and the cleaning of machines.
Environmental aspects
Chloroethanol is a metabolite in the degradation of 1,2-dichloroethane. The alcohol is then further oxidized via chloroacetaldehyde to chloroacetate. This metabolic pathway is topical since billions of kilograms of 1,2-dichloroethane are processed annually as a precursor to vinyl chloride.[10]
Safety
2-Chloroethanol is toxic with an -1">50 of 89 mg/kg in rats. Like most organochlorine compounds, chloroethanol releases hydrochloric acid and phosgene when burned.
In regards to dermal exposure to 2-chloroethanol, the Occupational Safety and Health Administration has set a permissible exposure limit of 5 ppm (16 mg/m3) over an eight-hour time-weighted average, while the National Institute for Occupational Safety and Health has a more protective recommended exposure limit of a 1 ppm (3 mg/m3) exposure ceiling.[11]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[12][failed verification]
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. 2014. p. 29. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. "For example, the omission of the locant ‘1’ in 2-chloroethanol, while permissible in general usage, is not allowed in preferred IUPAC names, thus the name 2-chloroethan-1-ol is the PIN."
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Depositor-supplied synonyms for CID 34.
- ↑ 3.0 3.1 3.2 3.3 NIOSH Pocket Guide to Chemical Hazards. "#0268". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0268.html.
- ↑ 4.0 4.1 "Ethylene chlorohydrin". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/107073.html.
- ↑ "NFPA Chemicals". http://www.newenv.com/resources/nfpa_chemicals.
- ↑ Ethylene chlorohydrin: properties
- ↑ 7.0 7.1 7.2 Liu, Gordon Y. T.; Richey, W. Frank; Betso, Joanne E.; Hughes, Brian; Klapacz, Joanna; Lindner, Joerg (2014). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_565.pub2.
- ↑ Butler J; Kellogg R (1987). "Synthesis of Macrocyclic Sulfides Using Cesium Thiolates: 1,4,8,11-Tetrathiacyclotetradecane". Organic Syntheses 65 (150): 150. doi:10.15227/orgsyn.065.0150.
- ↑ Raue, Roderich; Corbett, John F. (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_383.
- ↑ Janssen, D. B.; van der Ploeg, J. R.; Pries, F. (1994). "Genetics and Biochemistry of 1,2-Dichloroethane Degradation". Biodegradation 5 (3–4): 249–57. doi:10.1007/BF00696463. PMID 7765836. https://pure.rug.nl/ws/files/14528144/1994BiodegradJanssen.pdf.
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards
- ↑ Code of Federal Regulations (July 1, 2008 ed.). Government Printing Office.
 |