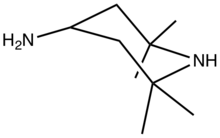
Chemistry:4-Amino-2,2,6,6-tetramethylpiperidine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2,6,6-Tetramethylpiperidin-4-amine | |
| Other names
2,2,6,6-Tetramethyl-4-aminopiperidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H20N2 | |
| Molar mass | 156.273 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.8966 g/cm3 |
| Melting point | 17 °C (63 °F; 290 K) |
| Boiling point | 188.5 °C (371.3 °F; 461.6 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H290, H302, H314, H412 | |
| P234, P260, P264, P270, P273, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P390, P404, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Amino-2,2,6,6-tetramethyl-4-piperidine is an organic compound with the formula H2NCH(CH2CMe2)2NH (where Me = CH3). Classified as a diamine, it is a colorless oily liquid.
The compound is an intermediate in the preparation of Bobbitt's salt, an oxidant used in organic synthesis. It is prepared by the reductive amination of the corresponding ketone:[1]
- OC(CH2CMe2)2NH + NH3 + H2 → H2NCH(CH2CMe2)2NH + H2O
Compound Properties
Boiling point is 188.5 °C [2] Melting point is 17 °C. [3] Density is 0.8966 g/cm3 @ Temp: 20 °C [4]
Toxicity
A study by Truda et al, has reported the median lethal dose LD(50) as 906mg/kg in rats. .[5]
References
- ↑ Nabyl Merbouh; James M. Bobbitt; Christian Brückner (2004). "Preparation of Tetramethylpiperdine-1-oxoammonlum Salts and Their Use as Oxidants in Organic Chemistry. A Review". Organic Preparations and Procedures International 36: 1–31. doi:10.1080/00304940409355369.
- ↑ [PhysProp data were obtained from: https://commonchemistry.cas.org/detail?cas_rn=36768-62-4]
- ↑ [PhysProp data were obtained from: https://commonchemistry.cas.org/detail?cas_rn=36768-62-4]
- ↑ [PhysProp data were obtained from: https://commonchemistry.cas.org/detail?cas_rn=36768-62-4]
- ↑ [ Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(5), Pg. 53, 1984]
Related compounds
Further reading
- C. McFarland; D. Vicic; A. Debnath (2006). "Rapid Microwave-Assisted Syntheses of Derivatives of HIV-1 Entry Inhibitors". Synthesis 2006 (5): 807–812. doi:10.1055/s-2006-926339.
- L. Tilley; J. Bobbitt; S. Murray; C. Camire (2013). "A Revised Preparation of (4-Acetamido-2,2,6,6-tetramethylpiperidin-1-yl)oxyl and 4-Acetamido-2,2,6,6-tetramethyl-1-oxopiperidinium Tetrafluoroborate: Reagents for Stoichiometric Oxidations of Alcohols". Synthesis 45 (3): 326–329. doi:10.1055/s-0032-1317861.
- A. Germanova; L. Aizvert; A. Shidlovskaia; L. Melnikova; M. Bidevkina (1984). "Comparative characteristics of the toxicity, safety and nature of the biological effect on the body of 4-hydroxy-2,2,6,6-tetramethylpiperidine and 4-amino-2,2,6,6-tetramethylpiperidine". Gig Tr Prof Zabol (5): 53–54. PMID 6745687.
- M. Gergely; A. Takacs; L.Kollar (2016). "4-Amino-TEMPO as N-Nucleophile in Palladium-Catalyzed Aminocarbonylation". Heterocyclic Chemistry 54 (1): 634–640. doi:10.1002/jhet.2635.
 |

