Chemistry:4-Methyl-2-pentanol
From HandWiki
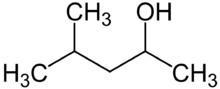
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methylpentan-2-ol | |
| Other names
4-Methyl-2-pentanol
Methyl isobutyl carbinol MIBC Isobutyl methyl carbinol 2-Methyl-4-pentanol 4-Methylpentane-2-ol 1,3-Dimethylbutanol Methyl amyl alcohol Isobutyl methyl methanol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2053 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.174 g/mol |
| Appearance | colorless liquid |
| Odor | mild |
| Density | 0.8075 g/cm3 at 20 °C |
| Melting point | −90 °C (−130 °F; 183 K) |
| Boiling point | 131.6 °C (268.9 °F; 404.8 K) |
| 15 g/L | |
| Solubility | soluble in ethanol, diethyl ether |
| Vapor pressure | 0.698 kPa |
| -80.4·10−6 cm3/mol | |
| Viscosity | 4.07 mPa·s |
| Thermochemistry | |
Heat capacity (C)
|
273.0 J·mol−1·K−1 (liquid) |
Std enthalpy of
formation (ΔfH⦵298) |
-394.7 kJ·mol−1 (liquid) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H226, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+361+353, P304+340, P312, P370+378, P403+233, P403+235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 41 °C (106 °F; 314 K) |
| Explosive limits | 1-5.5%[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2590 mg/kg (rat, oral)[3] |
LDLo (lowest published)
|
1000 mg/kg (mouse, oral)[3] |
LC50 (median concentration)
|
2000 ppm (rat, 4 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 25 ppm (100 mg/m3) [skin][2] |
REL (Recommended)
|
TWA 25 ppm (100 mg/m3) ST 40 ppm (165 mg/m3) [skin][2] |
IDLH (Immediate danger)
|
400 ppm[2] |
| Related compounds | |
Related compounds
|
Hexanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Methyl-2-pentanol (IUPAC name: 4-methylpentan-2-ol) or methyl isobutyl carbinol (MIBC) is an organic chemical compound used primarily as a frother in mineral flotation and in the production of lubricant oil additives such as Zinc dithiophosphate.[4] It is also used as a solvent, in organic synthesis, and in the manufacture of brake fluid[5] and as a precursor to some plasticizers. It is an acetone derivative in liquid state, with limited solubility in water but generally miscible with most organic solvents.[4]
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3-398, 5-47, 8-106, 15-22, 16-24, ISBN 0-8493-0594-2
- ↑ 2.0 2.1 2.2 2.3 NIOSH Pocket Guide to Chemical Hazards. "#0422". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0422.html.
- ↑ 3.0 3.1 3.2 "Methyl isobutyl carbinol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/108112.html.
- ↑ 4.0 4.1 "Methyl Isobutyl Carbinol (MIBC): Product Overview". https://www.celanese.com/products/mibc-methyl-isobutyl-carbinol/.
- ↑ Howard, Philip H. (1993), Handbook of Environmental Fate and Exposure Data for Organic Chemicals, 4, Boca Raton, Florida: CRC Press, pp. 430–434, ISBN 978-0-87371-413-6, https://books.google.com/books?id=HdhohbQrg8IC&pg=PA392, retrieved 2010-01-22
 |


