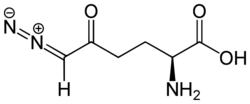
Chemistry:6-Diazo-5-oxo-L-norleucine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C6H9N3O3 |
| Molar mass | 171.156 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
6-Diazo-5-oxo-L-norleucine (DON) is a glutamine antagonist, which was isolated originally from Streptomyces in a sample of Peruvian soil. This diazo compound is biosynthesized from lysine by three enzymes in bacteria.[2] It is one of the most famous non-proteinogenic amino acid and was characterized in 1956 by Henry W Dion et al.,[3] who suggested a possible use in cancer therapy. This antitumoral efficacy was confirmed in different animal models.[4] DON was tested as chemotherapeutic agent in different clinical studies, but was never approved. In 2019, DON was shown to kill tumor cells while reversing disease symptoms and improve overall survival in late-stage experimental glioblastoma in mice, when combined with calorie-restricted ketogenic diet.[5]
Chemistry
DON is a water-soluble yellowish powder, which can be dissolved also in aqueous solutions of methanol, acetone or ethanol, but dissolution in absolute alcohols is difficult. Solutions of at least 50 μM DON in 0.9% NaCl are lightly yellowish. The crystalline form appears as yellowish greenish needles. The specific rotation is [α]26D +21° (c = 5.4% in H2O). In phosphate buffer, pH 7 are the ultraviolet absorption maxima at 274 nm (E1%1 cm. 683) and 244 nm (E1%1 cm 376).[3][6]
Biochemistry
DON is used as inhibitor of different glutamine utilizing enzymes. Due to its similarity to glutamine it can enter catalytic centres of these enzymes and inhibits them by covalent binding, or more precisely by alkylation.[7][8] The following table gives a survey of DON targets.
| Enzyme | Metabolic pathway | References |
|---|---|---|
| Carbamoyl phosphate synthase (CAD) | Pyrimidine-De-Novo-Synthesis | [7][9] |
| CTP synthase (CTPS) | Pyrimidine-De-Novo-Synthesis | [7][9] |
| FGAR amidotransferase | Purine-De-Novo-Synthesis | [7][10] |
| Guanosine monophosphate synthetase (GMPS) | Purine-De-Novo-Synthesis | [7][11] |
| PRPP amidotransferase | Purine-De-Novo-Synthesis | [7][11] |
| Mitochondrial glutaminase | First step of glutaminolysis | [7][11] |
| NAD synthase | Coenzyme of the electron transport chain | [7][12] |
| Asparagine synthetase | Amino acid synthesis | [7][13] |
Pharmacology
DON is a cytotoxic inhibitor of many enzymes of nucleotide synthesis. It could be shown in vitro that DON treatment led to apoptosis, the programmed cell death. Different pathways were investigated. So it could be shown that the inner mitochondrial membrane was damaged,[14] and single strand DNA breaks were observed.[15] The exact mode of action remains unclear and needs further research.
DON is not approved as pharmaceutical agent, but is tested in combination with a recombinant glutaminase in clinical trials for the treatment of different solid tumors.[16]
See also
- JHU-083, a prodrug of 6-diazo-5-oxo-L-norleucine
References
- ↑ "CID 5359375". PubChem. United States National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5359375&loc=ec_rcs.
- ↑ "Complete Biosynthetic Pathway of Alazopeptin, a Tripeptide Consisting of Two Molecules of 6-Diazo-5-oxo-l-norleucine and One Molecule of Alanine". Angewandte Chemie 60 (18): 10319–10325. April 2021. doi:10.1002/anie.202100462. PMID 33624374.
- ↑ 3.0 3.1 6-diazo-5-oxo-L-norleucine, A new tumor inhibitory substance. II: Isolation and Characterization. Antibiotics and Chemotherapy. 78. 1954. pp. 3075–7.
- ↑ "Glutamine antagonist with diet deficient in glutamine and aspartate reduce tumor growth". The Tokushima Journal of Experimental Medicine 39 (1–2): 69–76. June 1992. PMID 1412455.
- ↑ "Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma". Communications Biology 2 (1): 200. 29 May 2019. doi:10.1038/s42003-019-0455-x. PMID 31149644.
- ↑ "6-Diazo-5-oxo-L-norleucine, a New Tumor-inhibitory Substance.1a Preparation of L-, D- and DL-Forms1b". Journal of the American Chemical Society 80 (15): 3941–3945. August 1958. doi:10.1021/ja01548a036.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 "Glutamine binding sites". Affinity labeling. Methods in Enzymology. 46. 1977. pp. 414–27. doi:10.1016/S0076-6879(77)46049-X. ISBN 978-0-12-181946-0.
- ↑ "Reactions of Pseudomonas 7A glutaminase-asparaginase with diazo analogues of glutamine and asparagine result in unexpected covalent inhibitions and suggests an unusual catalytic triad Thr-Tyr-Glu". Biochemistry 39 (6): 1199–1204. February 2000. doi:10.1021/bi991797d. PMID 10684596.
- ↑ 9.0 9.1 "Pyrimidine Studies: I. Effect of DON (6-Diazo-5-oxo-l-norleucine) on Incorporation of Precursors into Nucleic Acid Pyrimidines". Cancer Research 18 (1): 105–109. 1 January 1958. https://cancerres.aacrjournals.org/content/18/1/105.
- ↑ "Biosynthesis of the purines. XV. The effect of aza-L-serine and 6-diazo-5-oxo-L-norleucine on inosinic acid biosynthesis de novo". The Journal of Biological Chemistry 225 (1): 163–176. March 1957. doi:10.1016/S0021-9258(18)64919-1. PMID 13416227.
- ↑ 11.0 11.1 11.2 "Metabolism and action of amino acid analog anti-cancer agents". Pharmacology & Therapeutics 46 (2): 243–271. 1990. doi:10.1016/0163-7258(90)90094-I. PMID 2108451. https://zenodo.org/record/1258343.
- ↑ "Effects of 6-diazo-5-oxol-norleucine and other tumor inhibitors on the biosynthesis of nicotinamide adenine dinucleotide in mice". Cancer Research 26 (2): 282–286. February 1966. PMID 4285554. http://cancerres.aacrjournals.org/cgi/reprint/26/2_Part_1/282.
- ↑ "DON, CONV and DONV-II. Inhibition of L-'asparagine synthetase in vivo". Biochemical Pharmacology 25 (16): 1851–1858. August 1976. doi:10.1016/0006-2952(76)90189-1. PMID 9091.
- ↑ "A mechanism behind the antitumour effect of 6-diazo-5-oxo-L-norleucine (DON): disruption of mitochondria". European Journal of Cancer 35 (7): 1155–1161. July 1999. doi:10.1016/S0959-8049(99)00099-4. PMID 10533463.
- ↑ "DNA strand cleavage by tumor-inhibiting antibiotic 6-diazo-5-oxo-L-norleucine". Mutation Research 360 (2): 95–100. June 1996. doi:10.1016/0165-1161(95)00073-9. PMID 8649470.
- ↑ "A phase IIa study of PEGylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors". J Clin Oncol 26 (May 20 Suppl): abstr 2533. 2008. doi:10.1200/jco.2008.26.15_suppl.2533.
 |
