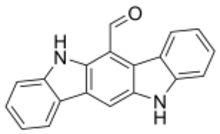
Chemistry:6-Formylindolo(3,2-b)carbazole
| |
| Names | |
|---|---|
| Preferred IUPAC name
5,11-Dihydroindolo[3,2-b]carbazole-6-carbaldehyde | |
| Other names
6-Formylindolo[3,2-b]carbazole
FICZ | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H12N2O | |
| Molar mass | 284.318 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
6-Formylindolo[3,2-b]carbazole (FICZ) is a chemical compound with the molecular formula C19H12N2O. It is a nitrogen heterocycle, having an extremely high affinity (Kd = 7 x 10−11M)[1][2] for binding to the aryl hydrocarbon receptor (AHR).
It was originally identified as a photooxidized derivative of the amino acid tryptophan and suggested to be the endogenous ligand of the AHR.[1] Later, also enzymatic formation of FICZ was shown.[3]
Occurrence
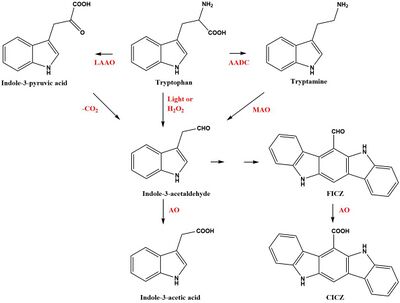
FICZ can be found in batches of tryptophan (Trp) and in any solution, including cell culture media, containing Trp, especially if exposed to ultraviolet light or visible light.[4][5][6] In human keratinocytes (HaCaT cells) grown in Trp-enriched medium and then irradiated with UVB formation of intracellular FICZ could also be demonstrated.[7] Similarly, FICZ has been identified and quantified in Jurkat cells grown in L-Trp enriched medium.[8] FICZ was first identified in humans as sulfoconjugates, a type of metabolites of FICZ, by use of liquid chromatography–mass spectrometry, (LC/MS/MS).[6] FICZ has been identified in the skin of persons with the disease vitiligo,[9] and in extracts of skin originating from patients with the Malassezia-associated diseases seborrhoeic dermatitis (SD) or pityriasis versicolor (PV).[10] Malassezia yeasts are commensal microorganisms found on the skin of many animals including humans. When the yeast stain Malassezia furfur is cultured on agar containing Trp as the only nitrogen source it produces FICZ and a variety of other indole derivatives .[11][12][3] The gastrointestinal tract is a rich source of microorganisms and a favorable environment for the formation of indoles and indole derivatives. Although FICZ itself has not been convincingly identified in the mouse colon, the precursors of FICZ e.g. indole-3-pyruvate, indole-3-acetaldehyde (I3A), and tryptamine, have been found.[13]
Biosynthesis and mechanisms of formation
In addition to the light- or H2O2-induced formation of FICZ, a number of other enzymatic pathways have been identified to convert Trp to FICZ via the precursor I3A.[2][3] Oxidative deamination of Trp by aromatic amino acid aminotransferases (ArAT) or L-amino oxidases (LAAO), one of which is the IL4-inducible enzyme IL4I1, converts Trp to indole-3-pyruvate, which after decarboxylation yields I3A. The enzyme aromatic L-amino acid decarboxylase (AADC) yields tryptamine via decarboxylation of Trp. Tryptamine can be oxidatively deaminated by monoamine oxidase (MAO-A and B) to produce I3A. Post incubation after deamination of the reaction mixture in the absence of an active enzyme generated FICZ and its oxidation product indolo[3,2-b]carbazole-6-carboxylic acid (CICZ). These non-enzymatic reactions were favored by low pH or increased temperature.[3]
Chemical syntheses
The indolo[3,2-b]carbazoles[14] have been intensely studied as synthetic targets due to their diverse biological effects and numerous applications in materials chemistry. The double Fischer indolization for the synthesis of the parent system indolo[3,2-b]carbazole was first reported by Robinson 1963[15] and has since been applied for the synthesis of FICZ and related structures.[14] A more practical and gram-scale synthesis of FICZ has been reported [16] using readily available and commercially obtainable starting materials such as 1-(phenylsulfonyl)-1H-indole and 1-(phenylsulfonyl)-1H-indole-3-carbaldehyde. In order to reach gram amounts in the multistep synthesis of this low solubility ring-closed carbazole (FICZ) the final purification by column chromatography was replaced by a crystallization step instead.
AHR-binding and gene induction
When the high-affinity AHR ligand FICZ binds to the receptor, which is a ligand dependent transcription factor, activation of many target genes takes place. The most well-studied of these target genes is the cytochrome P450 (CYP) 1A1. The CYPs are a superfamily of enzymes involved in the metabolism of a large number of both endogenous and exogenous compounds. The first chemical compound to be recognized as a high affinity AHR ligand was 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The CYP-induction by TCDD is sustained while the induction by FICZ is transient because of its rapid metabolic degradation by CYP1A1.[17][18]
Metabolism
In the first analyses of the metabolism of FICZ, human recombinant CYP1A1 enzyme and S9 fractions prepared from rat liver and mouse Hepa-1 cells were used.[17][18] Three HPLC fractions representing FICZ metabolites were occurring with time in the treated S9 derived from the wild type cells while no metabolites were detected in the S9 from CYP1A1-deficient cells.[18] In the further analyses of the CYP1A1 derived metabolites two monohydroxylated FICZ metabolites (2- and 8-hydroxyindolo[3,2-b]carbazole-6-carboxaldehyde) and three dihydroxylated metabolites (2,8-, 2,10-, and 4,8-dihydroxyindolo[3,2-b]carbazole-6-carboxaldehyde) were identified by LC-MS and nuclear magnetic resonance spectroscopy (NMR spectroscopy) and the chemical structure of the metabolites were confirmed by chemical analyses.[19] Kinetic studies using human recombinant CYP1A1, -1A2, and -1B1 expressed in E. coli showed that the catalytic efficiencies (specificity constant kcat/Km) were 5–50-fold higher for FICZ than for the standard substrates 7-ethoxyresorufin and 7-metoxyresorufin and at least 5000-fold higher compared with the hydroxylation of estrogens.[6] The catalytic efficiency for FICZ as a CYP1A1 substrate (kcat/Km of 8.1x107 M−1 s−1) is close to the limit of diffusion and thus FICZ is an excellent CYP1A1 substrate, but is also a very good substrate for CYP1A2 and CYP1B1.[6] The mono- and di-hydroxylated metabolites of FICZ are subject to further metabolic changes to glucuronide- and sulfate-conjugates.[20] Sulfotransferase (SULT)-catalyzed reactions resulted in a more pronounced reduction of the hydroxylated FICZ metabolites than the glucuronidation. Further studies using human recombinant SULTs showed that SULT1A1, -1A2, -1B1, and -1E1 exhibited high catalytic efficiencies and 2-hydroxylated FICZ was more efficiently conjugated than the 8-hydroxylated FICZ. With a kcat/Km of 1.1x107 M−1 s−1 with 2-OH-FICZ as substrate SULT1A2 exhibited a higher value than for any other substrate. Also dihydroxylated FICZ metabolites are converted to disulfuric acid esters but at a slower rate with intermediate formation of monosulfoconjugates, one of which was identified in human urine.[6]
The FICZ/AHR/CYP1A1 feedback loop
Already in the 1980s Nebert and coworkers proposed that a feedback loop involving an endogenous AHR ligand that is also a substrate for CYP1A1 regulates this signaling.[21] FICZ inhibits CYP1A1 activity, but the inhibition is transient since FICZ is such an exceptionally good substrate for the CYP1A1 enzyme and thereby generates a regulatory AHR feedback loop.[17] There are several substances, both exogenous and endogenous, which can inhibit CYP1A1 and indirectly leading to an accumulation of FICZ in the cell and a subsequent activation of the AHR and induction of CYP1A1.[22] This feedback system is essential for the physiological function of the AHR signaling, since the AHR regulates the balance between the quiescence and proliferation of a large number of cells, such as intra-thymic progenitor cells, as well as hematopoietic, pulmonary, and neuro-epithelial stem cells.[23]
Physiological functions mediated by FICZ
Self-renewal and differentiation of stem/progenitor cells
The AHR seems to play important roles in normal embryonic development and reversible repression of the receptor is essential for the maintenance of the pluripotency of embryonic stem cells (ESC).[24] It has been shown that the expansion of early progenitor murine hematopoietic stem cells is promoted by down-regulation of AHR signaling through the RNA-binding protein Musashi-2 and 200 nM FICZ reversed this effect.[25] Furthermore, the expansion of human induced pluripotent stem cells (iPSC) was enhanced by the AHR inhibitor CH223191.[26] In contrast, by applying a novel, pluripotent stem-cell-based in vitro culture system, it was demonstrated that the potent AhR ligand FICZ resulted in an exponential expansion (600-fold increase) of iPSC-derived hematopoietic progenitor cell (HP) populations.[27] Furthermore, FICZ treatment for extended periods (60 days) resulted in a progressive erythroid specification and maturation of the HP cells.[27] Various effects of the AHR and FICZ in cancer cells and cancer stem cells (CSCs) have also been described.[23]
Immune responses
The AHR is involved in the regulation of T helper 17 cell (Th17) and regulatory T cell (Treg) differentiation, which is of importance for the treatment of autoimmunity, infections and cancer. AHR activation by FICZ can promote the development of Th17 cells causing inflammation and autoimmunity, but also promote an expansion of the Treg cell population and thereby stimulate immunosuppressive activities.[28][29] Taking rapid metabolic degradation of FICZ into account, there seems to be no intrinsic difference in the effects of FICZ and TCDD on T cell differentiation and T cell-mediated adaptive immune responses as originally reported. Similarly, FICZ can stimulate or inhibit cytokine production and the maturation and homeostasis of mast cells in vitro, as well as anaphylactic responses in vivo, depending on the dose and timing of exposure.[23]
Immune barrier homeostases
The AHR is highly expressed in cells of the immune barrier organs, such as skin, lung, gut, and mucosal epithelia, as well as in the placenta.[30] AHR-deficient mice have fewer intestinal innate lymphoid cells (ILCs), which are the dominant source of interleukin 22 (IL-22) and they do not survive infection by the intestinal pathogen Citrobacter rodentium.[31] The same authors reported that in wild-type mice, FICZ increased the production of IL-22 by the ILCs.[31] In another study, daily i.p. injections of 100 μg x kg−1 FICZ to adult mice dramatically reduced their mortality following infection with the intestinal pathogen Listeria monocytogenes.[32] Stockinger and co-workers showed that efficient metabolic clearance of FICZ in mice that overexpress Cyp1a1 in the gut epithelium led to a pseudo-AHR-deficient state and when infected with Citrobacter rodentium, these animals exhibited markedly reduced numbers of group 3 ILCs and Th17 cells and succumbed rapidly.[33] Conversely, in mice lacking CYP1A1, or when CYP1A1 was inhibited, increased protection against intestinal infection was observed.[33] The composition of the murine commensal microbiota influences susceptibility to gastrointestinal infections and induced colitis and specific components of this microbiota promote the production of AHR ligands resulting in protection against intestinal damage induced by dextran sulfate sodium (DSS).[34] Monteleone and co-workers reported that mice injected with FICZ were protected against colitis in several experimental models of colitis.[35] Moreover, administration of anti-IL-22 prevented the anti-inflammatory effect of FICZ, demonstrating that the therapeutic effect of FICZ at least is partially mediated by IL-22.[35] The effect of AhR ligands in reversing inflammatory responses was also demonstrated in a clinical setting. FICZ treatment of lamina propria mononuclear cells from Crohn’s disease patients resulted in decreased IFN-γ expression and up-regulation of IL-22.[35] Lamas and co-workers showed that mice with dysbiotic microbiota due to their lack of the caspase recruitment domain 9 (CARD9) produced lower levels of endogenous AHR agonists and recovered more poorly from DSS-induced colitis. When 1 µg FICZ was injected i.p. one day after DSS administration, the severity of colitis in these animals was reduced significantly. Also, the defective colonic expression of IL-22 and genes coding for antimicrobial proteins in Card9 KO mice could be reversed by FICZ.[36]
FICZ seems also to be involved in the physiological regulation of Th2-mediated immunity in the lung. In vitro, FICZ markedly inhibited the lipopolysaccharide- and ovalbumin-induced proliferation of T cells.[37] FICZ likewise suppressed pulmonary Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma.[38]
Dynamic AHR signaling plays key roles in skin immunity. When full-thickness biopsies from the lesional skin of patients with psoriasis were exposed to FICZ, 29 genes belonging to the psoriasis transcriptome were down-regulated.[39] A later murine study confirmed that FICZ decreased IL-17 expression and lessened the severity of psoriasis.[40] Similarly, FICZ and AHR may be involved in the etiology of cutaneous systemic lupus erythematosus and atopic dermatitis.[41][42] There are also evidence for an important physiological role for FICZ in the expression of IL-22 in the skin. The uptake of Trp and intracellular accumulation of FICZ in skin γδ T cells is regulated by the activation marker CD69 in combination with the aromatic-amino-acid-transporter complex LAT1-CD98. These results revealed the importance of Trp uptake for AHR dependent secretion of IL-22 by γδ T cells during the development of psoriasis.[8]
The toxicity of FICZ
High levels of FICZ can exert ROS-dependent toxicity, whereas low levels can transiently elevate local levels of ROS/Ca2+, thereby promoting cellular adaptation, survival, and proliferation.[43] FICZ has proven to be potently embryotoxic toward fish and birds.[44][45] Zebrafish embryos demonstrated a dramatically increased mortality and severe toxicity of FICZ when CYP1A1 was inhibited.[44] FICZ is also an endogenous chromophore that at nanomolar concentrations potentiate photooxidative stress.[46] FICZ phototoxicity can be a valuable tool in elimination of skin cancer cells [47]
References
- ↑ 1.0 1.1 Rannug, A; Rannug, U; Rosenkranz, HS; Winqvist, L; Westerholm, R; Agurell, E; Grafström, AK (15 November 1987). "Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances.". The Journal of Biological Chemistry 262 (32): 15422–7. doi:10.1016/S0021-9258(18)47743-5. PMID 2824460.
- ↑ 2.0 2.1 Rannug, U; Rannug, A; Sjöberg, U; Li, H; Westerholm, R; Bergman, J (December 1995). "Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands.". Chemistry & Biology 2 (12): 841–5. doi:10.1016/1074-5521(95)90090-x. PMID 8807817.
- ↑ 3.0 3.1 3.2 3.3 3.4 Smirnova, A; Wincent, E; Vikström Bergander, L; Alsberg, T; Bergman, J; Rannug, A; Rannug, U (19 January 2016). "Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ.". Chemical Research in Toxicology 29 (1): 75–86. doi:10.1021/acs.chemrestox.5b00416. PMID 26686552.
- ↑ Oberg, M; Bergander, L; Håkansson, H; Rannug, U; Rannug, A (June 2005). "Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity.". Toxicological Sciences 85 (2): 935–43. doi:10.1093/toxsci/kfi154. PMID 15788723.
- ↑ Diani-Moore, S; Labitzke, E; Brown, R; Garvin, A; Wong, L; Rifkind, AB (March 2006). "Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo.". Toxicological Sciences 90 (1): 96–110. doi:10.1093/toxsci/kfj065. PMID 16330490.
- ↑ 6.0 6.1 6.2 6.3 6.4 Wincent, E; Amini, N; Luecke, S; Glatt, H; Bergman, J; Crescenzi, C; Rannug, A; Rannug, U (30 January 2009). "The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans.". The Journal of Biological Chemistry 284 (5): 2690–6. doi:10.1074/jbc.M808321200. PMID 19054769.
- ↑ Fritsche, E; Schäfer, C; Calles, C; Bernsmann, T; Bernshausen, T; Wurm, M; Hübenthal, U; Cline, JE et al. (22 May 2007). "Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation.". Proceedings of the National Academy of Sciences of the United States of America 104 (21): 8851–6. doi:10.1073/pnas.0701764104. PMID 17502624. Bibcode: 2007PNAS..104.8851F.
- ↑ 8.0 8.1 Cibrian, Danay; Saiz, María Laura; de la Fuente, Hortensia; Sánchez-Díaz, Raquel; Moreno-Gonzalo, Olga; Jorge, Inmaculada; Ferrarini, Alessia; Vázquez, Jesús et al. (August 2016). "CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis". Nature Immunology 17 (8): 985–996. doi:10.1038/ni.3504. ISSN 1529-2916. PMID 27376471.
- ↑ Schallreuter, KU; Salem, MA; Gibbons, NC; Maitland, DJ; Marsch, E; Elwary, SM; Healey, AR (June 2012). "Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 2: Epidermal H2O2/ONOO(-)-mediated stress in vitiligo hampers indoleamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immune response signaling.". FASEB Journal 26 (6): 2471–85. doi:10.1096/fj.11-201897. PMID 22415306.
- ↑ Magiatis, Prokopios; Pappas, Periklis; Gaitanis, George; Mexia, Nikitia; Melliou, Eleni; Galanou, Maria; Vlachos, Christophoros; Stathopoulou, Konstantina et al. (August 2013). "Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin". The Journal of Investigative Dermatology 133 (8): 2023–2030. doi:10.1038/jid.2013.92. ISSN 1523-1747. PMID 23448877.
- ↑ Gaitanis, G; Magiatis, P; Hantschke, M; Bassukas, ID; Velegraki, A (January 2012). "The Malassezia genus in skin and systemic diseases.". Clinical Microbiology Reviews 25 (1): 106–41. doi:10.1128/CMR.00021-11. PMID 22232373.
- ↑ Magiatis, P; Pappas, P; Gaitanis, G; Mexia, N; Melliou, E; Galanou, M; Vlachos, C; Stathopoulou, K et al. (August 2013). "Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin.". The Journal of Investigative Dermatology 133 (8): 2023–30. doi:10.1038/jid.2013.92. PMID 23448877.
- ↑ Agus, Allison; Planchais, Julien; Sokol, Harry (13 June 2018). "Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease". Cell Host & Microbe 23 (6): 716–724. doi:10.1016/j.chom.2018.05.003. ISSN 1934-6069. PMID 29902437.
- ↑ 14.0 14.1 Janosik, Tomasz; Rannug, Agneta; Rannug, Ulf; Wahlström, Niklas; Slätt, Johnny; Bergman, Jan (26 September 2018). "Chemistry and Properties of Indolocarbazoles". Chemical Reviews 118 (18): 9058–9128. doi:10.1021/acs.chemrev.8b00186. ISSN 1520-6890. PMID 30191712.
- ↑ Robinson, B. (1963). "568. The Fischer indolisation of cyclohexane-1,4-dione bisphenylhydrazone" (in en). Journal of the Chemical Society (Resumed): 3097–3099. doi:10.1039/jr9630003097. ISSN 0368-1769. http://xlink.rsc.org/?DOI=jr9630003097.
- ↑ Zhang, Cunyu; Creech, Katrina L.; Zuercher, William J.; Willson, Timothy M. (10 July 2019). "Gram-scale synthesis of FICZ, a photoreactive endogenous ligand of the aryl hydrocarbon receptor" (in en). Scientific Reports 9 (1): 9982. doi:10.1038/s41598-019-46374-7. ISSN 2045-2322. PMID 31292477. Bibcode: 2019NatSR...9.9982Z.
- ↑ 17.0 17.1 17.2 Wei, Y. D.; Helleberg, H.; Rannug, U.; Rannug, A. (12 March 1998). "Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole". Chemico-Biological Interactions 110 (1–2): 39–55. doi:10.1016/s0009-2797(97)00111-7. ISSN 0009-2797. PMID 9566724.
- ↑ 18.0 18.1 18.2 Wei, Y. D.; Bergander, L.; Rannug, U.; Rannug, A. (2000). "Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole". Archives of Biochemistry and Biophysics 383 (1): 99–107. doi:10.1006/abbi.2000.2037. ISSN 0003-9861. PMID 11097181.
- ↑ Bergander, L; Wahlström, N; Alsberg, T; Bergman, J; Rannug, A; Rannug, U (February 2003). "Characterization of in vitro metabolites of the aryl hydrocarbon receptor ligand 6-formylindolo[3,2-b]carbazole by liquid chromatography-mass spectrometry and NMR.". Drug Metabolism and Disposition: The Biological Fate of Chemicals 31 (2): 233–41. doi:10.1124/dmd.31.2.233. PMID 12527705.
- ↑ Bergander, Linda; Wincent, Emma; Rannug, Agneta; Foroozesh, Maryam; Alworth, William; Rannug, Ulf (2004). "Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole". Chemico-Biological Interactions 149 (2–3): 151–164. doi:10.1016/j.cbi.2004.08.005. ISSN 0009-2797. PMID 15501436.
- ↑ Hankinson, O; Andersen, RD; Birren, BW; Sander, F; Negishi, M; Nebert, DW (10 February 1985). "Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line". The Journal of Biological Chemistry 260 (3): 1790–5. doi:10.1016/S0021-9258(18)89662-4. PMID 3968086.
- ↑ Wincent, Emma; Bengtsson, Johanna; Mohammadi Bardbori, Afshin; Alsberg, Tomas; Luecke, Sandra; Rannug, Ulf; Rannug, Agneta (2012). "Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor". Proceedings of the National Academy of Sciences of the United States of America 109 (12): 4479–4484. doi:10.1073/pnas.1118467109. ISSN 1091-6490. PMID 22392998. Bibcode: 2012PNAS..109.4479W.
- ↑ 23.0 23.1 23.2 Rannug, Agneta; Rannug, Ulf (August 2018). "The tryptophan derivative 6-formylindolo[3,2-b]carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation". Critical Reviews in Toxicology 48 (7): 555–574. doi:10.1080/10408444.2018.1493086. ISSN 1547-6898. PMID 30226107.
- ↑ Ko, Chia-I.; Wang, Qin; Fan, Yunxia; Xia, Ying; Puga, Alvaro (January 2014). "Pluripotency factors and Polycomb Group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells". Stem Cell Research 12 (1): 296–308. doi:10.1016/j.scr.2013.11.007. ISSN 1876-7753. PMID 24316986.
- ↑ Rentas, Stefan; Holzapfel, Nicholas; Belew, Muluken S; Pratt, Gabriel; Voisin, Veronique; Wilhelm, Brian T; Bader, Gary D; Yeo, Gene W et al. (2016-04-28). "Musashi-2 Attenuates AHR Signaling to Expand Human Hematopoietic Stem Cells". Nature 532 (7600): 508–511. doi:10.1038/nature17665. ISSN 0028-0836. PMID 27121842. Bibcode: 2016Natur.532..508R.
- ↑ Leung, Amy; Zulick, Elizabeth; Skvir, Nicholas; Vanuytsel, Kim; Morrison, Tasha A.; Naing, Zaw Htut; Wang, Zhongyan; Dai, Yan et al. (July 2018). "Notch and Aryl Hydrocarbon Receptor Signaling Impact Definitive Hematopoiesis from Human Pluripotent Stem Cells". Stem Cells (Dayton, Ohio) 36 (7): 1004–1019. doi:10.1002/stem.2822. ISSN 1066-5099. PMID 29569827.
- ↑ 27.0 27.1 Smith, Brenden W.; Rozelle, Sarah S.; Leung, Amy; Ubellacker, Jessalyn; Parks, Ashley; Nah, Shirley K.; French, Deborah; Gadue, Paul et al. (2013-07-18). "The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation". Blood 122 (3): 376–385. doi:10.1182/blood-2012-11-466722. ISSN 1528-0020. PMID 23723449.
- ↑ Duarte, João H.; Di Meglio, Paola; Hirota, Keiji; Ahlfors, Helena; Stockinger, Brigitta (2013). "Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo". PLOS ONE 8 (11): e79819. doi:10.1371/journal.pone.0079819. ISSN 1932-6203. PMID 24244565. Bibcode: 2013PLoSO...879819D.
- ↑ Ehrlich, Allison K.; Pennington, Jamie M.; Bisson, William H.; Kolluri, Siva K.; Kerkvliet, Nancy I. (1 February 2018). "TCDD, FICZ, and Other High Affinity AhR Ligands Dose-Dependently Determine the Fate of CD4+ T Cell Differentiation". Toxicological Sciences 161 (2): 310–320. doi:10.1093/toxsci/kfx215. ISSN 1096-0929. PMID 29040756.
- ↑ Esser, Charlotte; Rannug, Agneta (April 2015). Ma, Qiang. ed. "The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology" (in en). Pharmacological Reviews 67 (2): 259–279. doi:10.1124/pr.114.009001. ISSN 0031-6997. PMID 25657351.
- ↑ 31.0 31.1 J, Qiu; Jj, Heller; X, Guo; Zm, Chen; K, Fish; Yx, Fu; L, Zhou (27 January 2012). "The Aryl Hydrocarbon Receptor Regulates Gut Immunity Through Modulation of Innate Lymphoid Cells" (in en). Immunity 36 (1): 92–104. doi:10.1016/j.immuni.2011.11.011. PMID 22177117.
- ↑ A, Kimura; H, Abe; S, Tsuruta; S, Chiba; Y, Fujii-Kuriyama; T, Sekiya; R, Morita; A, Yoshimura (April 2014). "Aryl Hydrocarbon Receptor Protects Against Bacterial Infection by Promoting Macrophage Survival and Reactive Oxygen Species Production" (in en). International Immunology 26 (4): 209–20. doi:10.1093/intimm/dxt067. PMID 24343818.
- ↑ 33.0 33.1 Schiering, Chris; Wincent, Emma; Metidji, Amina; Iseppon, Andrea; Li, Ying; Potocnik, Alexandre J.; Omenetti, Sara; Henderson, Colin J. et al. (2017). "Feedback control of AHR signalling regulates intestinal immunity". Nature 542 (7640): 242–245. doi:10.1038/nature21080. ISSN 1476-4687. PMID 28146477. Bibcode: 2017Natur.542..242S.
- ↑ Rannug, Agneta (2020-08-08). "How the AHR Became Important in Intestinal Homeostasis-A Diurnal FICZ/AHR/CYP1A1 Feedback Controls Both Immunity and Immunopathology". International Journal of Molecular Sciences 21 (16): 5681. doi:10.3390/ijms21165681. ISSN 1422-0067. PMID 32784381.
- ↑ 35.0 35.1 35.2 Monteleone, Ivan; Rizzo, Angelamaria; Sarra, Massimiliano; Sica, Giuseppe; Sileri, Pierpaolo; Biancone, Livia; MacDonald, Thomas T.; Pallone, Francesco et al. (July 2011). "Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract". Gastroenterology 141 (1): 237–248, 248.e1. doi:10.1053/j.gastro.2011.04.007. ISSN 1528-0012. PMID 21600206. https://www.openaccessrepository.it/record/23222.
- ↑ Lamas, Bruno; Richard, Mathias L.; Leducq, Valentin; Pham, Hang-Phuong; Michel, Marie-Laure; Da Costa, Gregory; Bridonneau, Chantal; Jegou, Sarah et al. (June 2016). "CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands". Nature Medicine 22 (6): 598–605. doi:10.1038/nm.4102. ISSN 1546-170X. PMID 27158904.
- ↑ Thatcher, Thomas H.; Williams, Marc A.; Pollock, Stephen J.; McCarthy, Claire E.; Lacy, Shannon H.; Phipps, Richard P.; Sime, Patricia J. (January 2016). "Endogenous ligands of the aryl hydrocarbon receptor regulate lung dendritic cell function". Immunology 147 (1): 41–54. doi:10.1111/imm.12540. ISSN 1365-2567. PMID 26555456.
- ↑ Jeong, Kyu-Tae; Hwang, Sung-Jun; Oh, Gap-Soo; Park, Joo-Hung (August 2012). "FICZ, a tryptophan photoproduct, suppresses pulmonary eosinophilia and Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma". International Immunopharmacology 13 (4): 377–385. doi:10.1016/j.intimp.2012.04.014. ISSN 1878-1705. PMID 22561122. https://pubmed.ncbi.nlm.nih.gov/22561122.
- ↑ Di Meglio, Paola; Duarte, João H.; Ahlfors, Helena; Owens, Nick D. L.; Li, Ying; Villanova, Federica; Tosi, Isabella; Hirota, Keiji et al. (19 June 2014). "Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions". Immunity 40 (6): 989–1001. doi:10.1016/j.immuni.2014.04.019. ISSN 1097-4180. PMID 24909886.
- ↑ Smith, Susan H.; Jayawickreme, Channa; Rickard, David J.; Nicodeme, Edwige; Bui, Thi; Simmons, Cathy; Coquery, Christine M.; Neil, Jessica et al. (October 2017). "Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans". The Journal of Investigative Dermatology 137 (10): 2110–2119. doi:10.1016/j.jid.2017.05.004. ISSN 1523-1747. PMID 28595996.
- ↑ Dorgham, Karim; Amoura, Zahir; Parizot, Christophe; Arnaud, Laurent; Frances, Camille; Pionneau, Cédric; Devilliers, Hervé; Pinto, Sandra et al. (November 2015). "Ultraviolet light converts propranolol, a nonselective β-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand". European Journal of Immunology 45 (11): 3174–3187. doi:10.1002/eji.201445144. ISSN 1521-4141. PMID 26354876.
- ↑ Kiyomatsu-Oda, Mari; Uchi, Hiroshi; Morino-Koga, Saori; Furue, Masutaka (June 2018). "Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis". Journal of Dermatological Science 90 (3): 284–294. doi:10.1016/j.jdermsci.2018.02.014. ISSN 1873-569X. PMID 29500077.
- ↑ Mohammadi-Bardbori, Afshin; Bastan, Farzaneh; Akbarizadeh, Amin-Reza (October 2017). "The highly bioactive molecule and signal substance 6-formylindolo[3,2-bcarbazole (FICZ) plays bi-functional roles in cell growth and apoptosis in vitro"]. Archives of Toxicology 91 (10): 3365–3372. doi:10.1007/s00204-017-1950-9. ISSN 1432-0738. PMID 28289825. https://pubmed.ncbi.nlm.nih.gov/28289825.
- ↑ 44.0 44.1 Wincent, Emma; Kubota, Akira; Timme-Laragy, Alicia; Jönsson, Maria E.; Hahn, Mark E.; Stegeman, John J. (2016). "Biological effects of 6-formylindolo[3,2-bcarbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner"]. Biochemical Pharmacology 110-111: 117–129. doi:10.1016/j.bcp.2016.04.012. ISSN 1873-2968. PMID 27112072.
- ↑ Jönsson, Maria E.; Mattsson, Anna; Shaik, Siraz; Brunström, Björn (January 2016). "Toxicity and cytochrome P450 1A mRNA induction by 6-formylindolo[3,2-bcarbazole (FICZ) in chicken and Japanese quail embryos"]. Comparative Biochemistry and Physiology. Toxicology & Pharmacology 179: 125–136. doi:10.1016/j.cbpc.2015.09.014. ISSN 1532-0456. PMID 26456929. https://pubmed.ncbi.nlm.nih.gov/26456929.
- ↑ Park, Sophia L.; Justiniano, Rebecca; Williams, Joshua D.; Cabello, Christopher M.; Qiao, Shuxi; Wondrak, Georg T. (June 2015). "The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-bCarbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes"]. The Journal of Investigative Dermatology 135 (6): 1649–1658. doi:10.1038/jid.2014.503. ISSN 1523-1747. PMID 25431849.
- ↑ Justiniano, Rebecca; de Faria Lopes, Lohanna; Perer, Jessica; Hua, Anh; Park, Sophia L.; Jandova, Jana; Baptista, Maurício S.; Wondrak, Georg T. (January 2021). "The Endogenous Tryptophan-derived Photoproduct 6-formylindolo[3,2-bcarbazole (FICZ) is a Nanomolar Photosensitizer that Can be Harnessed for the Photodynamic Elimination of Skin Cancer Cells in Vitro and in Vivo"]. Photochemistry and Photobiology 97 (1): 180–191. doi:10.1111/php.13321. ISSN 1751-1097. PMID 32767762.

