Chemistry:Astaxanthin
Astaxanthin /æstəˈzænθɪn/ is a keto-carotenoid within a group of chemical compounds known as carotenoids or terpenes.[1][2][3] Astaxanthin is a metabolite of zeaxanthin and canthaxanthin, containing both hydroxyl and ketone functional groups.[1]
It is a lipid-soluble pigment with red coloring properties, which result from the extended chain of conjugated (alternating double and single) double bonds at the center of the compound.[1] The presence of the hydroxyl functional groups and the hydrophobic hydrocarbons render the molecule amphiphilic.[4]
Astaxanthin is produced naturally in the freshwater microalgae Haematococcus pluvialis, the yeast fungus Xanthophyllomyces dendrorhous (also known as Phaffia rhodozyma) and the bacteria Paracoccus carotinifaciens. [5][6] When the algae are stressed by lack of nutrients, increased salinity, or excessive sunshine, they create astaxanthin.[7] Animals who feed on the algae, such as salmon, red trout, red sea bream, flamingos, and crustaceans (shrimp, krill, crab, lobster, and crayfish), subsequently reflect the red-orange astaxanthin pigmentation.[1][8]
Astaxanthin is used as a dietary supplement for human, animal, and aquaculture consumption.[1] Astaxanthin from algae, synthetic and bacterial sources is generally recognized as safe in the United States.[9] The US Food and Drug Administration has approved astaxanthin as a food coloring (or color additive) for specific uses in animal and fish foods.[1][10] The European Commission considers it as a food dye with E number E161j.[11] The European Food Safety Authority has set an Acceptable Daily Intake of 0.2 mg per kg body weight, as of 2019.[12] As a food color additive, astaxanthin and astaxanthin dimethyldisuccinate are restricted for use in Salmonid fish feed only.[13]
Natural sources


Astaxanthin is present in most red-coloured aquatic organisms.[8] The content varies from species to species, but also from individual to individual as it is highly dependent on diet and living conditions.[14] Astaxanthin and other chemically related asta-carotenoids have also been found in a number of lichen species of the Arctic zone.[citation needed]
The primary natural sources for industrial production of astaxanthin comprise the following:[8]
- Euphausia pacifica (Pacific krill)
- Euphausia superba (Antarctic krill)
- Haematococcus pluvialis (algae)
- Pandalus borealis (Arctic shrimp)
| Source | Astaxanthin concentration (ppm) |
|---|---|
| Salmonids | ~ 5 |
| Plankton | ~ 60 |
| Krill | ~ 120 |
| Arctic shrimp (Pandalus borealis) | ~ 1,200 |
| Phaffia yeast | ~ 10,000 |
| Paracoccus carotinifaciens | ~ 21,000 |
| Haematococcus pluvialis | ~ 40,000 |
Algae are the primary natural source of astaxanthin in the aquatic food chain. The microalgae Haematococcus pluvialis contains high levels of astaxanthin (about 3.8% of dry weight), and is the primary industrial source of natural astaxanthin.[15]
In shellfish, astaxanthin is almost exclusively concentrated in the shells, with only low amounts in the flesh itself, and most of it only becomes visible during cooking as the pigment separates from the denatured proteins that otherwise bind it. Astaxanthin is extracted from Euphausia superba (Antarctic krill) and from shrimp processing waste.[16]
Biosynthesis
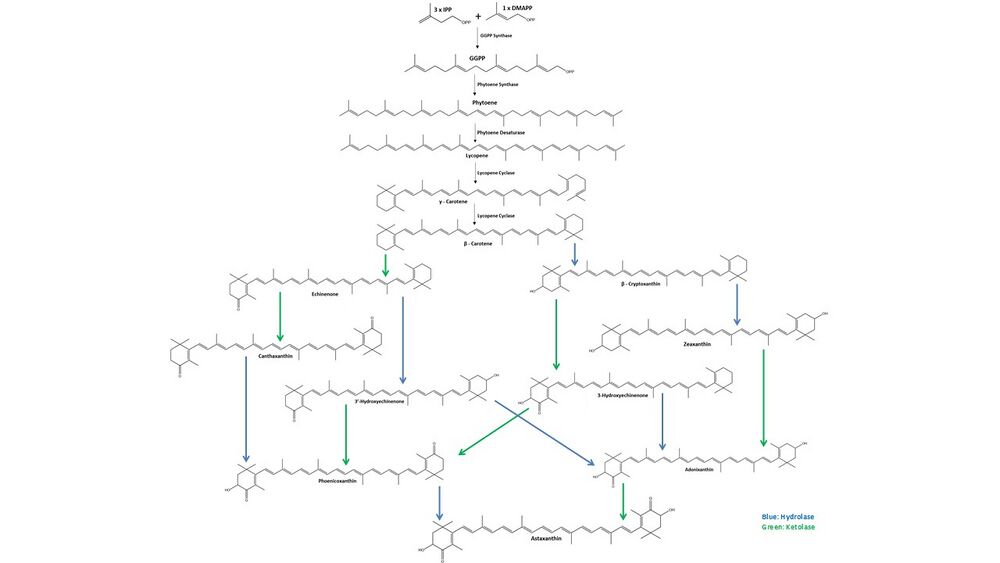
Astaxanthin biosynthesis starts with three molecules of isopentenyl pyrophosphate (IPP) and one molecule of dimethylallyl pyrophosphate (DMAPP) that are combined by IPP isomerase and converted to geranylgeranyl pyrophosphate (GGPP) by GGPP synthase. Two molecules of GGPP are then coupled by phytoene synthase to form phytoene. Next, phytoene desaturase creates four double bonds in the phytoene molecule to form lycopene. After desaturation, lycopene cyclase first forms γ-carotene by converting one of the ψ acyclic ends of the lycopene as a β-ring, then subsequently converts the other to form β-carotene. From β-carotene, hydrolases (blue) are responsible for the inclusion of two 3-hydroxy groups, and ketolases (green) for the addition of two 4-keto groups, forming multiple intermediate molecules until the final molecule, astaxanthin, is obtained.[17]
Synthetic sources
The structure of astaxanthin by synthesis was described in 1975.[18] Nearly all commercially available astaxanthin for aquaculture is produced synthetically, with an annual market of about $1 billion in 2019.[19]
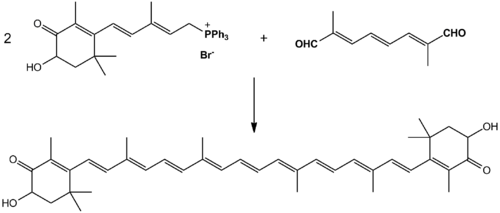
An efficient synthesis from isophorone, cis-3-methyl-2-penten-4-yn-1-ol and a symmetrical C10-dialdehyde has been discovered and is used in industrial production. It combines these chemicals together with an ethynylation and then a Wittig reaction.[20] Two equivalents of the proper ylide combined with the proper dialdehyde in a solvent of methanol, ethanol, or a mixture of the two, yields astaxanthin in up to 88% yields.[21]
Metabolic engineering
The cost of astaxanthin extraction, high market price, and lack of efficient fermentation production systems, combined with the intricacies of chemical synthesis, discourage its commercial development. The metabolic engineering of bacteria (Escherichia coli) enables efficient astaxanthin production from beta-carotene via either zeaxanthin or canthaxanthin.[1][22][23][24]
Structure
Stereoisomers
In addition to structural isomeric configurations, astaxanthin also contains two chiral centers at the 3- and 3′-positions, resulting in three unique stereoisomers (3R,3′R and 3R,3'S meso and 3S,3'S). While all three stereoisomers are present in nature, relative distribution varies considerably from one organism to another.[25] Synthetic astaxanthin contains a mixture of all three stereoisomers, in approximately 1:2:1 proportions.[26]
Esterification
Astaxanthin exists in two predominant forms, non-esterified (yeast, synthetic) or esterified (algal) with various length fatty acid moieties whose composition is influenced by the source organism as well as growth conditions. The astaxanthin fed to salmon to enhance flesh coloration is in the non-esterified form [27] The predominance of evidence supports a de-esterification of fatty acids from the astaxanthin molecule in the intestine prior to or concomitant with absorption resulting in the circulation and tissue deposition of non-esterified astaxanthin. European Food Safety Authority (EFSA) published a scientific opinion on a similar xanthophyll carotenoid, lutein, stating that "following passage through the gastrointestinal tract and/or uptake lutein esters are hydrolyzed to form free lutein again".[28] While it can be assumed that non-esterified astaxanthin would be more bioavailable than esterified astaxanthin due to the extra enzymatic steps in the intestine needed to hydrolyse the fatty acid components, several studies suggest that bioavailability is more dependent on formulation than configuration.[29][30]
Uses
Astaxanthin is used as a dietary supplement and feed supplement as food colorant for salmon, crabs, shrimp, chickens and egg production.[1][15]
For seafood and animals
The primary use of synthetic astaxanthin today is as an animal feed additive to impart coloration, including farm-raised salmon and chicken egg yolks.[1][31] Synthetic carotenoid pigments colored yellow, red or orange represent about 15–25% of the cost of production of commercial salmon feed.[32] In the 21st century, most commercial astaxanthin for aquaculture is produced synthetically.[33]
Class action lawsuits were filed against some major grocery store chains for not clearly labeling the astaxanthin-treated salmon as "color added".[34] The chains followed up quickly by labeling all such salmon as "color added". Litigation persisted with the suit for damages, but a Seattle judge dismissed the case, ruling that enforcement of the applicable food laws was up to government and not individuals.[35]
Dietary supplement
The primary human application for astaxanthin is as a dietary supplement, and it remains under preliminary research.[1] In 2020, the European Food Safety Authority reported that an intake of 8 mg astaxanthin per day from food supplements is safe for adults.[36]
Role in the food chain
Lobsters, shrimp, and some crabs turn red when cooked because the astaxanthin, which was bound to the protein in the shell, becomes free as the protein denatures and unwinds. The freed pigment is thus available to absorb light and produce the red color.[1][37]
Regulations
In April 2009, the United States Food and Drug Administration approved astaxanthin as an additive for fish feed only as a component of a stabilized color additive mixture. Color additive mixtures for fish feed made with astaxanthin may contain only those diluents that are suitable.[10] The color additives astaxanthin, ultramarine blue, canthaxanthin, synthetic iron oxide, dried algae meal, Tagetes meal and extract, and corn endosperm oil are approved for specific uses in animal foods.[38] Haematococcus algae meal (21 CFR 73.185) and Phaffia yeast (21 CFR 73.355) for use in fish feed to color salmonoids were added in 2000.[39][40][41] In the European Union, astaxanthin-containing food supplements derived from sources that have no history of use as a source of food in Europe, fall under the remit of the Novel Food legislation, EC (No.) 258/97. Since 1997, there have been five novel food applications concerning products that contain astaxanthin extracted from these novel sources. In each case, these applications have been simplified or substantial equivalence applications, because astaxanthin is recognised as a food component in the EU diet.[42][43][44][45]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Astaxanthin". PubChem, US National Library of Medicine. May 3, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/5281224.
- ↑ Margalith, P. Z. (1999). "Production of ketocarotenoids by microalgae". Applied Microbiology and Biotechnology 51 (4): 431–8. doi:10.1007/s002530051413. PMID 10341427.
- ↑ Choi, Seyoung; Koo, Sangho (2005). "Efficient Syntheses of the Keto-carotenoids Canthaxanthin, Astaxanthin, and Astacene". The Journal of Organic Chemistry 70 (8): 3328–31. doi:10.1021/jo050101l. PMID 15823009.
- ↑ Ahirwar, Ankesh (August 3, 2021). "Light modulates transcriptomic dynamics upregulating astaxanthin accumulation in Haematococcus: A review". Bioresource Technology 340. doi:10.1016/j.biortech.2021.125707. PMID 34371336. https://www.sciencedirect.com/science/article/pii/S0960852421010488. Retrieved November 15, 2023.
- ↑ "Phaffia rhodozyma M.W. Mill., Yoney. & Soneda - Names Record". Species Fungorum. http://www.speciesfungorum.org/Names/NamesRecord.asp?RecordID=319694.
- ↑ Tsubokura, Akira; Yoneda, Hisashi; Mizuta, Haruyoshi (January 1, 1999). "Paracoccus carotinifaciens sp. nov., a new aerobic Gram-negative astaxanthin-producing bacterium" (in en). International Journal of Systematic and Evolutionary Microbiology 49 (1): 277–282. doi:10.1099/00207713-49-1-277. ISSN 1466-5026. PMID 10028273. https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/00207713-49-1-277.
- ↑ Aizpuru, Aitor; González-Sánchez, Armando (July 20, 2024). "Traditional and new trend strategies to enhance pigment contents in microalgae" (in en). World Journal of Microbiology and Biotechnology 40 (9): 272. doi:10.1007/s11274-024-04070-3. ISSN 1573-0972. PMID 39030303.
- ↑ 8.0 8.1 8.2 "Biorefinery approach and environment-friendly extraction for sustainable production of astaxanthin from marine wastes". Critical Reviews in Biotechnology 39 (4): 469–488. June 2019. doi:10.1080/07388551.2019.1573798. PMID 30939937. https://www.tandfonline.com/doi/full/10.1080/07388551.2019.1573798.
- ↑ Astaxanthin wins full GRAS status. Nutraingredients-usa.com. Retrieved on April 25, 2013.
- ↑ 10.0 10.1 "Summary of Color Additives for Use in United States in Foods, Drugs, Cosmetics, and Medical Devices". March 4, 2022. https://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm115641.htm. See Note 1.
- ↑ E-numbers : E100- E200 Food Colours. Food-Info.net. Retrieved on April 25, 2013.
- ↑ Safety and efficacy of astaxanthin-dimethyldisuccinate (Carophyll Stay-Pink 10%-CWS) for salmonids, crustaceans and other fish European Food Safety Authority. Retrieved on August 24, 2020.
- ↑ Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. Fda.gov. Retrieved on January 16, 2019.
- ↑ Abd El-Ghany, Mohamed N.; Hamdi, Salwa A.; Elbaz, Reham M.; Aloufi, Abeer S.; El Sayed, Rana R.; Ghonaim, Ghadeer M.; Farahat, Mohamed G. (May 24, 2023). "Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste" (in en). Fermentation 9 (6): 505. doi:10.3390/fermentation9060505. ISSN 2311-5637.
- ↑ 15.0 15.1 "Astaxanthin: sources, extraction, stability, biological activities and its commercial applications--a review". Marine Drugs 12 (1): 128–52. January 2014. doi:10.3390/md12010128. PMID 24402174.
- ↑ Katevas, Dimitri Sclabos (October 6, 2003). The Krill. aquafeed.com
- ↑ Barredo, Jose; García-Estrada, Carlos; Kosalkova, Katarina; Barreiro, Carlos (July 30, 2017). "Biosynthesis of Astaxanthin as a Main Carotenoid in the Heterobasidiomycetous Yeast Xanthophyllomyces dendrorhous". Journal of Fungi 3 (3): 44. doi:10.3390/jof3030044. ISSN 2309-608X. PMID 29371561.
- ↑ Cooper, R. D. G.; Davis, J. B.; Leftwick, A. P.; Price, C.; Weedon, B. (1975). "Carotenoids and related compounds. XXXII. Synthesis of astaxanthin, hoenicoxanthin, hydroxyechinenone, and the corresponding diosphenols". J. Chem. Soc. Perkin Trans. 1 (21): 2195–2204. doi:10.1039/p19750002195.
- ↑ "Recent progress in practical applications of a potential carotenoid astaxanthin in aquaculture industry: a review". Fish Physiology and Biochemistry 50 (1): 97–126. February 2024. doi:10.1007/s10695-022-01167-0. PMID 36607534.
- ↑ Ashford's Dictionary of Industrial Chemicals, 3rd Edition, 2011, p. 984, ISBN 095226742X.
- ↑ Krause, Wolfgang; Henrich, Klaus; Paust, Joachim; et al. Preaparation of Astaxanthin. DE 19509955. March 9, 18, 1995
- ↑ Scaife, M. A.; Burja, A. M.; Wright, P. C. (2009). "Characterization of cyanobacterial β-carotene ketolase and hydroxylase genes inEscherichia coli, and their application for astaxanthin biosynthesis". Biotechnology and Bioengineering 103 (5): 944–955. doi:10.1002/bit.22330. PMID 19365869.
- ↑ Scaife, MA; Ma, CA; Ninlayarn, T; Wright, PC; Armenta, RE (May 22, 2012). "Comparative Analysis of β-Carotene Hydroxylase Genes for Astaxanthin Biosynthesis". Journal of Natural Products 75 (6): 1117–24. doi:10.1021/np300136t. PMID 22616944.
- ↑ Lemuth, K; Steuer, K; Albermann, C (April 26, 2011). "Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin". Microbial Cell Factories 10: 29. doi:10.1186/1475-2859-10-29. PMID 21521516.
- ↑ Bjerkeng, Bjørn (1997). "Chromatographic Analysis of Synthesized Astaxanthin—A Handy Tool for the Ecologist and the Forensic Chemist?". The Progressive Fish-Culturist 59 (2): 129–140. doi:10.1577/1548-8640(1997)059<0129:caosaa>2.3.co;2. ISSN 0033-0779.
- ↑ Stachowiak, Barbara; Szulc, Piotr (May 2, 2021). "Astaxanthin for the Food Industry". Molecules 26 (9): 2666. doi:10.3390/molecules26092666. PMID 34063189. "It is noteworthy that astaxanthin synthesized in nature occurs in the trans form (3S, 3S), whereas synthetic astaxanthin is a mixture of two optical isomers and the meso form at a ratio of 1:2:1 (3R, 30R), (3R, 30S) and (3S, 30S).".
- ↑ Rüfer, Corinna E.; Moeseneder, Jutta; Briviba, Karlis; Rechkemmer, Gerhard; Bub, Achim (2008). "Bioavailability of astaxanthin stereoisomers from wild (Oncorhynchus spp.) and aquacultured (Salmo salar) salmon in healthy men: a randomised, double-blind study". The British Journal of Nutrition 99 (5): 1048–54. doi:10.1017/s0007114507845521. ISSN 0007-1145. PMID 17967218.
- ↑ "Scientific Opinion on the re-evaluation of lutein preparations other than lutein with high concentrations of total saponified carotenoids at levels of at least 80%". EFSA Journal 9 (5): 2144. 2011. doi:10.2903/j.efsa.2011.2144. ISSN 1831-4732.
- ↑ Landrum, J; Bone, R; Mendez, V; Valenciaga, A; Babino, D (2012). "Comparison of dietary supplementation with lutein diacetate and lutein: a pilot study of the effects on serum and macular pigment.". Acta Biochimica Polonica 59 (1): 167–9. doi:10.18388/abp.2012_2198. ISSN 0001-527X. PMID 22428144.
- ↑ Norkus, EP; Norkus, KL; Dharmarajan, TS; Schierle, J; Schalch, W (2010). "Serum lutein response is greater from free lutein than from esterified lutein during 4 weeks of supplementation in healthy adults.". Journal of the American College of Nutrition 29 (6): 575–85. doi:10.1080/07315724.2010.10719896. ISSN 0731-5724. PMID 21677121.
- ↑ Shah, M. M; Liang, Y; Cheng, J. J; Daroch, M (2016). "Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products". Frontiers in Plant Science 7: 531. doi:10.3389/fpls.2016.00531. PMID 27200009.
- ↑ Fisheries and Oceans Canada – Aquaculture Issues. pac.dfo-mpo.gc.ca.
- ↑ Juan F Martín, Eduardo Gudiña and José L Barredo (February 20, 2008). "Conversion of β-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein?". Microbial Cell Factories 7: 3. doi:10.1186/1475-2859-7-3. PMID 18289382.
- ↑ "Farm-Raised Salmon Cases: Private Action for Violation of California State Law is Not Preempted by the FDC Act". 2008. https://www.thefdalawblog.com/2008/02/farm-raised-sal/.
- ↑ "Pigments in Salmon Aquaculture: How to Grow a Salmon-colored Salmon". http://www.seafoodmonitor.com/sample/salmon.html.
- ↑ Turck, Dominique; Knutsen, Helle Katrine; Castenmiller, Jacqueline; de Henauw, Stefaan; Hirsch-Ernst, Karen Ildico; Kearney, John; Maciuk, Alexandre; Mangelsdorf, Inge et al. (2020). "Safety of astaxanthin for its use as a novel food in food supplements". EFSA Journal 18 (2): 4. doi:10.2903/j.efsa.2020.5993. PMID 32874213.
- ↑ Begum, Shamima (2015). "On the origin and variation of colors in lobster carapace". Physical Chemistry Chemical Physics 17 (26): 16723–16732. doi:10.1039/C4CP06124A. PMID 25797168. Bibcode: 2015PCCP...1716723B.
- ↑ See 21 CFR 73.35,73.50, 73.75, 73.200, 73.275, 73.295, 73.315, respectively.
- ↑ Code of Federal Regulations Title 21 § 73.35 FDA decision on Astaxanthin. Accessdata.fda.gov. Retrieved on April 25, 2013.
- ↑ Code of Federal Regulations Title 21 § 73.185 FDA decision on Haematococcus algae meal. Accessdata.fda.gov. Retrieved on April 25, 2013.
- ↑ Food Additive Status List. fda.gov
- ↑ Astaxanthin extract. acnfp.food.gov.uk
- ↑ Astaxanthin extract: Cyanotech Corporation. acnfp.gov.uk
- ↑ Astaxanthin extract: Algatechnologies (1998) Ltd. acnfp.gov.uk
- ↑ Astaxanthin extract: Parry Nutraceuticals. acnfp.gov.uk
 |
