Chemistry:Tretinoin
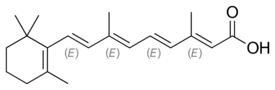 | |
| Clinical data | |
|---|---|
| Pronunciation | See pronunciation note |
| Trade names | Retin-a, Avita, Renova, others |
| Other names | ATRA |
| AHFS/Drugs.com | Monograph Topical Monograph |
| MedlinePlus | a608032 |
| License data | |
| Pregnancy category | |
| Routes of administration | Topical, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | > 95% |
| Elimination half-life | 0.5–2 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 180 °C (356 °F) |
| |
| |
| (verify) | |
Tretinoin, also known as all-trans retinoic acid (ATRA), is a medication used for the treatment of acne and acute promyelocytic leukemia.[6][7][8] For acne, it is applied to the skin as a cream, gel or ointment.[8] For leukemia, it is taken by mouth for up to three months.[6] Topical tretinoin is also the most extensively investigated retinoid therapy for photoaging.[9]
Common side effects when used as a cream are limited to the skin and include skin redness, peeling, and sun sensitivity.[8] When used by mouth, side effects include shortness of breath, headache, numbness, depression, skin dryness, itchiness, hair loss, vomiting, muscle pains, and vision changes.[6] Other severe side effects include high white blood cell counts and blood clots.[6] Use during pregnancy is contraindicated due to the risk of birth defects.[6][1] It is in the retinoid family of medications.[7]
Tretinoin was patented in 1957, and approved for medical use in 1962.[10] It is on the World Health Organization's List of Essential Medicines.[11] Tretinoin is available as a generic medication.[12] In 2020, it was the 230th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[13][14]
Medical uses
Skin use
Acne
Tretinoin is most commonly used to treat acne,[4] both inflammatory and noninflammatory. Multiple studies support the efficacy of topical retinoids in the treatment of acne vulgaris.[15][16] It is sometimes used in conjunction with other topical acne medications to enhance their penetration.[17] In addition to treating active acne, retinoids accelerate the resolution of acne-induced postinflammatory hyperpigmentation.[18] It is also useful as maintenance therapy for people who have responded well to their initial treatment, reducing the prolonged use of antibiotics for acne.[19]
Photoaging
Photoaging is premature skin aging resulting from prolonged and repeated exposure to solar radiation. Features of photoaging include fine and coarse wrinkles, change in skin pigmentation, and loss of elasticity. In human skin, topical retinoids increase collagen production, induce epidermal hyperplasia, and decrease keratinocyte and melanocyte atypia. Topical tretinoin is the most extensively investigated retinoid therapy for photoaging.[20] Topical tretinoin can be used for mild to severe photoaging in people of all skin types. Several weeks or months of use are typically required before improvement is appreciated. Although it has only been studied for a duration of two years, it may be continued indefinitely. A long-term maintenance regimen with a lower concentration or less frequent application may be an alternative to continued use.[21]
Leukemia
Tretinoin is used to induce remission in people with acute promyelocytic leukemia (APL) who have a mutation (the t(15;17) translocation that gives rise to the PML::RARα fusion gene). It is not used for maintenance therapy.[3][22][23]
The evidence is very uncertain about the effect of tretinoin in addition to chemotherapy for patients with non-APL acute myeloid leukemia on diarrhoea, nausea/vomiting and heart-related toxicity grades III/IV. Furthermore, tretinoin in addition to chemotherapy probably results in little to no difference in the mortality, relapse, progress, mortality during the trial and infections grade III/IV.[24]
Side effects
Skin use
Topical tretinoin is only for use on the skin and should not be applied to eyes or mucosal tissues. Common side effects include skin irritation, redness, swelling, and blistering.[4] If irritation is a problem, a decrease in the frequency of application to every other or every third night can be considered, and the frequency of application can be increased as tolerance improves. The fine skin flaking that is often seen can be gently exfoliated with a washcloth. A non-comedogenic facial moisturizer can also be applied if needed. Delaying application of the retinoid for at least 20 minutes after washing and drying the face may also be helpful. Topical retinoids are not true photosensitizing drugs, but people using topical retinoids have described symptoms of increased sun sensitivity. This is thought to be due to thinning of the stratum corneum leading to a decreased barrier against ultraviolet light exposure, as well as an enhanced sensitivity due to the presence of cutaneous irritation.[25]
Leukemia use
The oral form of the drug has boxed warnings concerning the risks of retinoic acid syndrome and leukocytosis.[3]
Other significant side effects include a risk of thrombosis, benign intracranial hypertension in children, high lipids (hypercholesterolemia and/or hypertriglyceridemia), and liver damage.[3]
There are many significant side effects from this drug that include malaise (66%), shivering (63%), hemorrhage (60%), infections (58%), peripheral edema (52%), pain (37%), chest discomfort (32%), edema (29%), disseminated intravascular coagulation (26%), weight increase (23%), injection site reactions (17%), anorexia (17%), weight decrease (17%), and myalgia (14%).[3]
Respiratory side effects usually signify retinoic acid syndrome, and include upper respiratory tract disorders (63%), dyspnea (60%), respiratory insufficiency (26%), pleural effusion (20%), pneumonia (14%), rales (14%), and expiratory wheezing (14%), and many others at less than 10%.[3]
Around 23% of people taking the drug have reported earache or a feeling of fullness in their ears.[3]
Gastrointestinal disorders include bleeding (34%), abdominal pain (31%), diarrhea (23%), constipation (17%), dyspepsia (14%), and swollen belly (11%) and many others at less than 10%.[3]
In the cardiovascular system, side effects include arrhythmias (23%), flushing (23%), hypotension (14%), hypertension (11%), phlebitis (11%), and cardiac failure (6%) and for 3% of patients: cardiac arrest, myocardial infarction, enlarged heart, heart murmur, ischemia, stroke, myocarditis, pericarditis, pulmonary hypertension, secondary cardiomyopathy.[3]
In the nervous system, side effects include dizziness (20%), paresthesias (17%), anxiety (17%), insomnia (14%), depression (14%), confusion (11%), and many others at less than 10% frequency.[3]
In the urinary system, side effects include chronic kidney disease (11%) and several others at less than 10% frequency.[3]
Mechanism of action
For its use in cancer, its mechanism of action is unknown, but on a cellular level, laboratory tests show that tretinoin forces APL cells to differentiate and stops them from proliferating; in people there is evidence that it forces the primary cancerous promyelocytes to differentiate and mature into neutrophils, allowing normal cells to repopulate the bone marrow.[3] A recent study showed that ATRA inhibits and degrades active PIN1.[26]
For its use in acne, tretinoin (along with other retinoids) are vitamin A derivatives that act by binding to two nuclear receptor families within keratinocytes: the retinoic acid receptors (RAR) and the retinoid X receptors (RXR).[18] These events contribute to the normalization of follicular keratinization and decreased cohesiveness of keratinocytes, resulting in reduced follicular occlusion and microcomedone formation.[27] The retinoid-receptor complex competes for coactivator proteins of AP-1, a key transcription factor involved in inflammation.[18] Retinoids also down-regulate expression of toll-like receptor (TLR)-2, which has been implicated in the inflammatory response in acne.[28] Moreover, tretinoin and retinoids may enhance the penetration of other topical acne medications.[17]
The combination of the 10% benzoyl peroxide and light results in more than 50% degradation of tretinoin in about 2 hours and 95% in 24 hours.[29] This lack of stability in the presence of light and oxidizing agents has led to the development of novel formulations of the drug. When microencapsulated tretinoin is exposed to benzoyl peroxide and light only 1% degradation takes place in about 4 hours and only 13% after 24 hours.[30]
Furthermore, studies have shown that tretinoin plays a regulatory role in the G1/S transition of neuroblastoma cells by influencing the activities of key kinases. Kinase-substrate enrichment analysis revealed increased CDK5 activity and reduced CDK2 activity during neuronal differentiation induced by tretinoin [31]. This alteration suggests that tretinoin influences multiple proteins involved in the G1/S transition, leading to the coordination of cell cycle arrest and inhibition of proliferation. By extending the duration of the G1 phase, tretinoin enables neuroblastoma cells to integrate environmental signals and respond to differentiation cues, promoting their differentiation and commitment to specialized cell fates instead of continued proliferation.
Biosynthesis
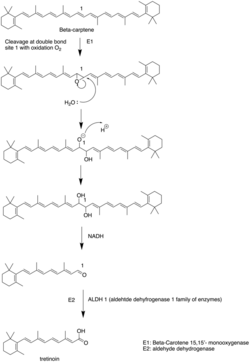
Tretinoin is synthesized from beta-carotene. The beta-carotene is firstly cleaved into beta-carotene 15-15'-monooxygenase through site 1 double bond oxidized to epoxide. The epoxide is attacked by water to form diol in site 1. NADH, as a reduction agent, reduce the alcohol group to aldehydes.[32]
History
Tretinoin was co-developed for its use in acne by James Fulton and Albert Kligman when they were at University of Pennsylvania in the 1960s.[33][34] Phase I trials, the first conducted on human subjects, were performed on inmates at Holmesburg Prison during a long-running regime of non-therapeutic and unethical testing on prison inmates at Holmesburg.[35][36] The University of Pennsylvania held the patent for Retin-A, which it licensed to pharmaceutical companies.[34]
Treatment of acute promyelocytic leukemia was first introduced at Ruijin Hospital in Shanghai by Wang Zhenyi in a 1988 clinical trial.[37]
Etymology
The origin of the name tretinoin is uncertain,[38][39] although several sources agree (one with probability,[38] one with asserted certainty[40]) that it probably comes from trans- + retinoic [acid] + -in, which is plausible given that tretinoin is the all-trans isomer of retinoic acid. The name isotretinoin is the same root tretinoin plus the prefix iso-. Regarding pronunciation, the following variants apply equally to both tretinoin and isotretinoin. Given that retinoic is pronounced /ˌrɛtɪˈnoʊɪk/,[39][40][41][42] it is natural that /ˌtrɛtɪˈnoʊɪn/ is a commonly heard pronunciation. Dictionary transcriptions also include /ˌtrɪˈtɪnoʊɪn/ (tri-TIN-oh-in)[39][41] and /ˈtrɛtɪnɔɪn/.[40][42]
Hair loss
Tretinoin has been explored as a treatment for hair loss, potentially as a way to increase the ability of minoxidil (by acting as an enzyme and accelerating the production of minoxidil sulfate) to penetrate the scalp, but the evidence is weak and contradictory.[43][44]
References
- ↑ 1.0 1.1 "Tretinoin (Vesanoid) Use During Pregnancy". 25 July 2019. https://www.drugs.com/pregnancy/tretinoin.html.
- ↑ "Tretinoin topical Use During Pregnancy". 1 July 2019. https://www.drugs.com/pregnancy/tretinoin-topical.html.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 "Tretinoin capsule". 12 December 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=264138c1-9e5f-45ef-be87-79ca97c989d9.
- ↑ 4.0 4.1 4.2 "Tretinoin Cream- tretinoin cream". 1 December 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=745cc3ce-60b4-4479-a055-316817567949.
- ↑ "List of nationally authorised medicinal products:Active substance(s): tretinoin (oral formulations)". European Medicines Agency. 1 December 2022. https://www.ema.europa.eu/en/documents/psusa/tretinoin-oral-formulations-list-nationally-authorised-medicinal-products-psusa/00003015/202203_en.pdf.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Tretinoin". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/tretinoin.html.
- ↑ 7.0 7.1 Resistance to Targeted Therapies Against Adult Brain Cancers. Springer. 2016. p. 123. ISBN 978-3-319-46505-0. https://books.google.com/books?id=XaCYDQAAQBAJ&pg=PA123.
- ↑ 8.0 8.1 8.2 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 627, 821–822. ISBN 978-0-85711-156-2.
- ↑ "Retinoids, topical". American Osteopathic College of Dermatology. https://www.aocd.org/page/Retinoidstopical.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 476. ISBN 978-3-527-60749-5. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA476.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Tretinoin topical". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/tretinoin-topical.html.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Tretinoin – Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Tretinoin.
- ↑ "Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment". Clinical Therapeutics 27 (2): 216–24. February 2005. doi:10.1016/j.clinthera.2005.02.009. PMID 15811485.
- ↑ "A combined analysis of 2 randomized clinical studies of tretinoin gel 0.05% for the treatment of acne". Cutis 83 (3): 146–54. March 2009. PMID 19363908.
- ↑ 17.0 17.1 "Management of acne: a report from a Global Alliance to Improve Outcomes in Acne". Journal of the American Academy of Dermatology 49 (1 Suppl): S1-37. July 2003. doi:10.1067/mjd.2003.618. PMID 12833004.
- ↑ 18.0 18.1 18.2 "Topical retinoids.". Fitzpatrick's Dermatology in General Medicine (7th ed.). New York: McGraw Hill. 2008. p. 2106.
- ↑ "Why Topical Retinoids Are Mainstay of Therapy for Acne". Dermatology and Therapy 7 (3): 293–304. September 2017. doi:10.1007/s13555-017-0185-2. PMID 28585191.
- ↑ "Photoaging". Dermatologic Clinics 32 (3): 291–9, vii. July 2014. doi:10.1016/j.det.2014.03.015. PMID 24891052.
- ↑ "Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial". American Journal of Clinical Dermatology 6 (4): 245–53. 2005. doi:10.2165/00128071-200506040-00005. PMID 16060712.
- ↑ "Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia". Blood 72 (2): 567–72. August 1988. doi:10.1182/blood.V72.2.567.567. PMID 3165295. http://www.bloodjournal.org/cgi/reprint/72/2/567.pdf.
- ↑ "All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results". Blood 76 (9): 1704–9. November 1990. doi:10.1182/blood.V76.9.1704.1704. PMID 2224119. http://www.bloodjournal.org/cgi/reprint/76/9/1704.pdf.
- ↑ "Effects of all-trans retinoic acid (ATRA) in addition to chemotherapy for adults with acute myeloid leukaemia (AML) (non-acute promyelocytic leukaemia (non-APL))". The Cochrane Database of Systematic Reviews 2018 (8): CD011960. August 2018. doi:10.1002/14651858.CD011960.pub2. PMID 30080246.
- ↑ "Topical retinoids in the treatment of acne vulgaris". Seminars in Cutaneous Medicine and Surgery 27 (3): 177–82. September 2008. doi:10.1016/j.sder.2008.06.001. PMID 18786495.
- ↑ "Active Pin1 is a key target of all-trans retinoic acid in acute promyelocytic leukemia and breast cancer". Nature Medicine 21 (5): 457–66. May 2015. doi:10.1038/nm.3839. PMID 25849135.
- ↑ Fernandez [Graber] EM, Zaenglein A, Thiboutot D. Acne Treatment Methodologies. In: Cosmetic Formulation of Skin Care Products, Taylor and Francis Group, New York 2006. p.273.
- ↑ "Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function". Journal of Immunology 174 (5): 2467–70. March 2005. doi:10.4049/jimmunol.174.5.2467. PMID 15728448.
- ↑ "Chemical stability of adapalene and tretinoin when combined with benzoyl peroxide in presence and in absence of visible light and ultraviolet radiation". The British Journal of Dermatology (Wiley) 139 (Suppl 52): 8–11. October 1998. doi:10.1046/j.1365-2133.1998.1390s2008.x. PMID 9990414.
- ↑ "The Stability of Tretinoin in Tretinoin Gel Microsphere 0.1%" (in en). https://www.mdedge.com/dermatology/article/66872/acne/stability-tretinoin-tretinoin-gel-microsphere-01.
- ↑ "Temporal Quantitative Proteomic and Phosphoproteomic Profiling of SH-SY5Y and IMR-32 Neuroblastoma Cells during All-Trans-Retinoic Acid-Induced Neuronal Differentiation". International Journal of Molecular Sciences 25 (2): 1047. January 2024. doi:10.3390/ijms25021047. PMID 38256121.
- ↑ "Oxidative cleavage of carotenoids catalyzed by enzyme models and beta-carotene 15,15´-monooxygenase". Pure and Applied Chemistry 74 (8): 1397–1408. 1 January 2002. doi:10.1351/pac200274081397.
- ↑ "Vivant Skin Care Co-founder James E. Fulton, MD, Loses Colon Cancer Battle". Vivant Pharmaceuticals, LLC Press Release. 10 July 2013. http://vivantskin.blogspot.com/2013/07/vivant-skin-care-co-founder-james-e.html.
- ↑ 34.0 34.1 "Dr. Albert M. Kligman, Dermatologist, Dies at 93". The New York Times. 22 February 2010. https://www.nytimes.com/2010/02/23/us/23kligman.html?_r=0.
- ↑ Medical apartheid : the dark history of medical experimentation on Black Americans from colonial times to the present. New York: Doubleday. 2006. ISBN 0-385-50993-6. OCLC 61131882. https://www.worldcat.org/oclc/61131882.
- ↑ Acres of skin : human experiments at Holmesburg Prison : a story of abuse and exploitation in the name of medical science. New York: Routledge. 1998. ISBN 0-415-91990-8. OCLC 37884781. https://www.worldcat.org/oclc/37884781.
- ↑ "Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia". Blood 72 (2): 567–72. August 1988. doi:10.1182/blood.V72.2.567.567. PMID 3165295.
- ↑ 38.0 38.1 Merriam-Webster's Unabridged Dictionary, Merriam-Webster, http://unabridged.merriam-webster.com/unabridged/, retrieved 12 July 2016.
- ↑ 39.0 39.1 39.2 Oxford Dictionaries Online, Oxford University Press, http://www.oxforddictionaries.com/.
- ↑ 40.0 40.1 40.2 Houghton Mifflin Harcourt, The American Heritage Dictionary of the English Language, Houghton Mifflin Harcourt, https://ahdictionary.com/, retrieved 24 January 2015.
- ↑ 41.0 41.1 Merriam-Webster's Medical Dictionary, Merriam-Webster, http://unabridged.merriam-webster.com/medical/.
- ↑ 42.0 42.1 Dorland's Illustrated Medical Dictionary, Elsevier, http://dorlands.com/.
- ↑ "Diagnosis and Treatment". The Difficult Hair Loss Patient: Guide to Successful Management of Alopecia and Related Conditions.. Cham: Springer. 2015. ISBN 978-3-319-19701-2. https://books.google.com/books?id=0ue5BAAAQBAJ&pg=PA95.
- ↑ "Medical treatments for male and female pattern hair loss". Journal of the American Academy of Dermatology 59 (4): 547–66; quiz 567–8. October 2008. doi:10.1016/j.jaad.2008.07.001. PMID 18793935.
External links
 |