Chemistry:Boron arsenide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| BAs | |
| Molar mass | 85.733 g/mol[1] |
| Appearance | Brown cubic crystals[1] |
| Density | 5.22 g/cm3[1] |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) decomposes[1] |
| Insoluble | |
| Band gap | 1.82 eV |
| Thermal conductivity | 1300 W/(m·K) (300 K) |
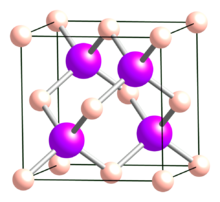
| Structure[2] | |
| Cubic (sphalerite), cF8, No. 216 | |
| F43m | |
a = 0.4777 nm
| |
Formula units (Z)
|
4 |
| Related compounds | |
Other anions
|
Boron nitride Boron phosphide Boron antimonide |
Other cations
|
Aluminium arsenide Gallium arsenide Indium arsenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| |
| Identifiers | |
|---|---|
| Properties | |
| B12As2 | |
| Molar mass | 279.58 g/mol |
| Density | 3.56 g/cm3[3] |
| Insoluble | |
| Band gap | 3.47 eV |
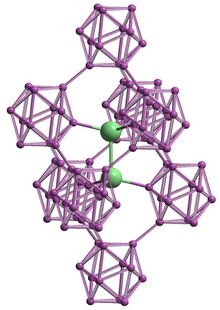
| Structure[4] | |
| Rhombohedral, hR42, No. 166 | |
| R3m | |
a = 0.6149 nm, b = 0.6149 nm, c = 1.1914 nm α = 90°, β = 90°, γ = 120°
| |
Formula units (Z)
|
6 |
| Related compounds | |
Other anions
|
Boron suboxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Boron arsenide (or Arsenic boride) is a chemical compound involving boron and arsenic, usually with a chemical formula BAs. Other boron arsenide compounds are known, such as the subarsenide B
12As
2. Chemical synthesis of cubic BAs is very challenging and its single crystal forms usually have defects.
Properties
BAs is a cubic (sphalerite) semiconductor in the III-V family with a lattice constant of 0.4777 nm and an indirect band gap of 1.82 eV. Cubic BAs is reported to decompose to the subarsenide B12As2 at temperatures above 920 °C.[5] Boron arsenide has a melting point of 2076 °C. The thermal conductivity of BAs is very high: around 1300 W/(m·K) at 300 K.
The basic physical properties of cubic BAs have been experimentally measured: Band gap (1.82 eV), optical refractive index (3.29 at wavelength 657 nm), elastic modulus (326 GPa), shear modulus, Poisson's ratio, thermal expansion coefficient (3.85×10−6/K), and heat capacity. It can be alloyed with gallium arsenide to produce ternary and quaternary semiconductors.[6]
BAs has high electron and hole mobility, >1000 cm2/V/second, unlike silicon which has high electron mobility, but low hole mobility.[7]
In 2023, a study in journal Nature reported that subjected to high high pressure BAs decrease its thermal conductivity contrary to the typical increase seen in most materials.[8][9][10]
Boron subarsenide
Boron arsenide also occurs as subarsenides, including the icosahedral boride B
12As
2. It belongs to R3m space group with a rhombohedral structure based on clusters of boron atoms and two-atom As–As chains. It is a wide-bandgap semiconductor (3.47 eV) with the extraordinary ability to "self-heal" radiation damage.[11] This form can be grown on substrates such as silicon carbide.[12] Another use for solar cell fabrication[6][13] was proposed, but it is not currently used for this purpose.
Applications
Boron arsenide is most attractive for use in electronics thermal management. Experimental integration with gallium nitride transistors to form GaN-BAs heterostructures has been demonstrated and shows better performance than the best GaN HEMT devices on silicon carbide or diamond substrates. Manufacturing BAs composites was developed as highly conducting and flexible thermal interfaces.[14]
First-principles calculations have predicted that the thermal conductivity of cubic BAs is remarkably high, over 2,200 W/(m·K) at room temperature, which is comparable to that of diamond and graphite.[15] Subsequent measurements yielded a value of only 190 W/(m·K) due to the high density of defects.[16][17] More recent first-principles calculations incorporating four-phonon scattering predict a thermal conductivity of 1400 W/(m·K).[18] Later, defect-free boron arsenide crystals have been experimentally realized and measured with an ultrahigh thermal conductivity of 1300 W/(m·K), consistent with theory predictions. Crystals with small density of defects have shown thermal conductivity of 900–1000 W/(m·K).[19][20]
The cubic-shaped boron arsenide has been discovered to be better at conducting heat and electricity than silicon, as well as reportedly better than silicon at conducting both electrons and its positively charged counterpart, the "electron-hole."[21]
References
- ↑ 1.0 1.1 1.2 1.3 Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.53. ISBN 1439855110.
- ↑ Perri, J. A; La Placa, S; Post, B (1958). "New group III-group V compounds: BP and BAs". Acta Crystallographica 11 (4): 310. doi:10.1107/S0365110X58000827.
- ↑ Villars, Pierre (ed.) "B12As2 (B6As) Crystal Structure" in Inorganic Solid Phases, Springer, Heidelberg (ed.) SpringerMaterials
- ↑ Morosin, B; Aselage, T. L; Feigelson, R. S (2011). "Crystal Structure Refinements of Rhombohedral Symmetry Materials Containing Boron-Rich Icosahedra". MRS Proceedings 97. doi:10.1557/PROC-97-145.
- ↑ Chu, T. L; Hyslop, A. E (1974). "Preparation and Properties of Boron Arsenide Films". Journal of the Electrochemical Society 121 (3): 412. doi:10.1149/1.2401826. Bibcode: 1974JElS..121..412C.
- ↑ 6.0 6.1 Geisz, J. F; Friedman, D. J; Olson, J. M; Kurtz, Sarah R; Reedy, R. C; Swartzlander, A. B; Keyes, B. M; Norman, A. G (2000). "BGaInAs alloys lattice matched to GaAs". Applied Physics Letters 76 (11): 1443. doi:10.1063/1.126058. Bibcode: 2000ApPhL..76.1443G.
- ↑ Shin, Jungwoo; Gamage, Geethal Amila; Ding, Zhiwei; Chen, Ke; Tian, Fei; Qian, Xin; Zhou, Jiawei; Lee, Hwijong et al. (2022-07-22). "High ambipolar mobility in cubic boron arsenide" (in en). Science 377 (6604): 437–440. doi:10.1126/science.abn4290. ISSN 0036-8075. PMID 35862526. Bibcode: 2022Sci...377..437S. https://www.science.org/doi/10.1126/science.abn4290.
- ↑ "Surprising heat transfer behaviour seen in new semiconductor under pressure" (in en-GB). 2023-01-27. https://physicsworld.com/surprising-heat-transfer-behaviour-seen-in-new-semiconductor-under-pressure/.
- ↑ Li, Suixuan; Qin, Zihao; Wu, Huan; Li, Man; Kunz, Martin; Alatas, Ahmet; Kavner, Abby; Hu, Yongjie (23 November 2022). "Anomalous thermal transport under high pressure in boron arsenide" (in en). Nature 612 (7940): 459–464. doi:10.1038/s41586-022-05381-x. ISSN 1476-4687. PMID 36418403. Bibcode: 2022Natur.612..459L. https://www.nature.com/articles/s41586-022-05381-x.
- ↑ Remmel, Ariana (2 January 2023). "Boron arsenide breaks the rules under pressure". C&EN 101 (1): 6. doi:10.1021/cen-10101-scicon3. https://cen.acs.org/materials/semiconductor-breaks-rules-physics-under/100/web/2022/12. Retrieved 2 April 2023.
- ↑ Carrard, M; Emin, D; Zuppiroli, L (1995). "Defect clustering and self-healing of electron-irradiated boron-rich solids". Physical Review B 51 (17): 11270–11274. doi:10.1103/PhysRevB.51.11270. PMID 9977852. Bibcode: 1995PhRvB..5111270C.
- ↑ Chen, H.; Wang, G.; Dudley, M.; Xu, Z.; Edgar, J. H.; Batten, T.; Kuball, M.; Zhang, L. et al. (2008). "Single-Crystalline B12As2 on m-plane (1100) 15R-SiC". Applied Physics Letters 92 (23): 231917. doi:10.1063/1.2945635. Bibcode: 2008ApPhL..92w1917C.
- ↑ Boone, J. L. and Vandoren, T. P. (1980) Boron arsenide thin film solar cell development, Final Report, Eagle-Picher Industries, Inc., Miami, OK. abstract.
- ↑ Cui, Ying; Qin, Zihao; Wu, Huan; Li, Man; Hu, Yongjie (2021). "Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management". Nature Communications 12 (1): 1284. doi:10.1038/s41467-021-21531-7. PMID 33627644. Bibcode: 2021NatCo..12.1284C..
- ↑ An unlikely competitor for diamond as the best thermal conductor, Phys.org news (July 8, 2013)
- ↑ Lv, Bing; Lan, Yucheng; Wang, Xiqu; Zhang, Qian; Hu, Yongjie; Jacobson, Allan J; Broido, David; Chen, Gang et al. (2015). "Experimental study of the proposed super-thermal-conductor: BAs". Applied Physics Letters 106 (7): 074105. doi:10.1063/1.4913441. Bibcode: 2015ApPhL.106g4105L. https://dspace.mit.edu/bitstream/1721.1/117852/1/Lv2015APL_BAs.pdf.
- ↑ Zheng, Qiang; Polanco, Carlos A.; Du, Mao-Hua; Lindsay, Lucas R.; Chi, Miaofang; Yan, Jiaqiang; Sales, Brian C. (6 September 2018). "Antisite Pairs Suppress the Thermal Conductivity of BAs". Physical Review Letters 121 (10): 105901. doi:10.1103/PhysRevLett.121.105901. PMID 30240242. Bibcode: 2018PhRvL.121j5901Z.
- ↑ Feng, Tianli; Lindsay, Lucas; Ruan, Xiulin (2017). "Four-phonon scattering significantly reduces intrinsic thermal conductivity of solids". Physical Review B 96 (16): 161201. doi:10.1103/PhysRevB.96.161201. Bibcode: 2017PhRvB..96p1201F.
- ↑ Li, Sheng; Zheng, Qiye; Lv, Yinchuan; Liu, Xiaoyuan; Wang, Xiqu; Huang, Pinshane Y.; Cahill, David G.; Lv, Bing (2018). "High thermal conductivity in cubic boron arsenide crystals". Science 361 (6402): 579–581. doi:10.1126/science.aat8982. PMID 29976796. Bibcode: 2018Sci...361..579L.
- ↑ Tian, Fei; Song, Bai; Chen, Xi; Ravichandran, Navaneetha K; Lv, Yinchuan; Chen, Ke; Sullivan, Sean; Kim, Jaehyun et al. (2018). "Unusual high thermal conductivity in boron arsenide bulk crystals". Science 361 (6402): 582–585. doi:10.1126/science.aat7932. PMID 29976797. Bibcode: 2018Sci...361..582T.
- ↑ General, Ryan (18 August 2022). "Chinese MIT professor helps discover 'game changer' months after espionage charges" (in en). NextShark. https://www.yahoo.com/news/chinese-mit-professor-helps-discover-215437385.html. Retrieved 19 August 2022.
External links
- 2020 paper by Malica and Dal Corso - Temperature dependent elastic constants and thermodynamic properties of BAs: An ab initio investigation
- Matweb data
- King, R. B. (1999). Boron Chemistry at the Millennium. New York: Elsevier. ISBN 0-444-72006-5.
- Ownby, P. D. (1975). "Ordered Boron Arsenide". Journal of the American Ceramic Society 58 (7–8): 359–360. doi:10.1111/j.1151-2916.1975.tb11514.x.
- High ambipolar mobility in cubic boron arsenide, Science
 |

