Chemistry:Hexaborane(10)
| |
| Identifiers | |
|---|---|
| |
| Properties | |
| B6H10 | |
| Molar mass | 74.94 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexaborane, also called hexaborane(10) to distinguish it from hexaborane(12) (B6H12), is an inorganic compound with the formula B6H10. It is a colorless liquid that is unstable in air.[1]
Structure
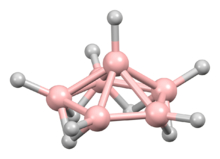
Hexaborane(10) is classified as a nido-cluster.[2]: 152 The boron atoms define a pentagonal pyramid, with four bridging hydrogen atoms and six terminal ones. The point group of the molecule is Cs.[3]
Preparation and reactions
A laboratory route begins with bromination of pentaborane(11) followed by deprotonation of the bromide to give [BrB5H7]−. This anionic cluster is reduced with diborane to give the neutral product:[1]
- K[BrB5H7] + 1/2 B2H6 → KBr + B6H10
It can also be generated by pyrolysis of pentaborane(11).
B6H10 can be deprotonated to give [B6H9]− or protonated to give [B6H11]+.[1] It can act as a Lewis base towards reactive borane radicals, forming various conjuncto-clusters.[2]: 162
References
- ↑ 1.0 1.1 1.2 Remmel, R. J.; Johnson, H. D.; Brice, V. T.; Shore, S. G.; Gaines, D. F. (1979). "Hexaborane(10)". Inorganic Syntheses 19: 247–253. doi:10.1002/9780470132500.ch58. ISBN 9780470132500.
- ↑ 2.0 2.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Hirshfeld, F. L.; Eriks, K.; Dickerson, R. E.; Lippert, E. L.; Lipscomb, William N. (1958). "Molecular and Crystal Structure of B6H10" (in en). The Journal of Chemical Physics 28 (1): 56–61. doi:10.1063/1.1744080. ISSN 0021-9606. Bibcode: 1958JChPh..28...56H. http://aip.scitation.org/doi/10.1063/1.1744080.
 |







