Chemistry:Boron triazide
| |
| Names | |
|---|---|
| IUPAC name
Triazidoborane
| |
| Other names
Triazidoborane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
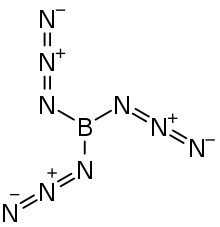
| B(N 3) 3 | |
| Molar mass | 136.87 g/mol |
| Appearance | colorless crystals |
| Solubility | soluble in diethyl ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Boron triazide, also known as triazidoborane, is a thermally unstable compound of boron and nitrogen with a nitrogen content of 92.1 % (by the standard atomic weight). Formally, it is the triazido derivative of borane and is a covalent inorganic azide. The high-energy compound, which has the propensity to undergo spontaneous explosive decomposition, was first described in 1954 by Egon Wiberg and Horst Michaud of the University of Munich.[1]
Preparation
The first method is by the addition of diborane to a solution of hydrazoic acid in diethyl ether at a temperature range between −20 °C and −10 °C. This synthesis proceeds via the intermediates monoazidoborane, BH
2N
3, and diazidoborane, BH(N
3)
2.[1]
- B
2H
6 + 6 HN
3 → 2 B(N
3)
3 + 6 H
2
The compound can also be obtained by passing boron tribromide vapor over solid silver azide in high vacuum.[2]
- BBr
3 + 3 AgN
3 → B(N
3)
3 + 3 AgBr
A similar gas-phase synthesis uses the spontaneous reaction of boron trichloride with hydrazoic acid.[3][4]
- BCl
3 + 3 HN
3 → B(N
3)
3 + 3 HCl
Properties
The compound forms colorless crystals that are only stable at low temperatures. Above −35 °C, an explosive decomposition may occur.[1] In the gas phase, generated boron triazide decomposes at room temperature within 60 minutes via loss of nitrogen gas to form boron nitrides with formulas BN
3 and BN. These reactions can also be initiated photochemically by UV radiation in the compounds absorption range at about 230 nm.[3][4][5]
- B(N
3)
3 → BN
3 + 3 N
2 - B(N
3)
3 → BN + 4 N
2
In contact with water, it undergoes hydrolysis to hydrazoic acid and boron trioxide.[3]
- 2 B(N
3)
3 + 3 H
2O → 6 HN
3 + B
2O
3
Reaction with other azides like sodium azide or lithium azide yields the corresponding tetraazidoborate complexes.[1][6]
- B(N
3)
3 + NaN
3 → Na[B(N
3)
4] - B(N
3)
3 + LiN
3 → Li[B(N
3)
4]
The parent tetraazidoboric acid, H[B(N
3)
4], can be obtained at temperatures lower than −60 °C.[1]
Uses
Due to the low stability, the compound itself is not used as a high-energy substance. However, the tetraazidoborate derivatives and adducts with bases such as quinoline, pyrazine or 2,2,6,6-tetramethylpiperidine have potential for this usage.[7] The gas-phase decomposition of the compound is also of interest as a method of coating surfaces with boron nitride.[3]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Wiberg, Egon; Michaud, Horst (1954-07-01). "Notizen: Zur Kenntnis eines Bortriazids B(N3)3". Zeitschrift für Naturforschung B 9 (7): 497–499. doi:10.1515/znb-1954-0715. ISSN 1865-7117.
- ↑ Liu, Fengyi; Zeng, Xiaoqing; Zhang, Jianping; Meng, Lingpeng; Zheng, Shijun; Ge, Maofa; Wang, Dianxun; Kam Wah Mok, Daniel et al. (2006). "A simple method to generate B(N3)3" (in en). Chemical Physics Letters 419 (1–3): 213–216. doi:10.1016/j.cplett.2005.11.082. Bibcode: 2006CPL...419..213L. https://linkinghub.elsevier.com/retrieve/pii/S0009261405018129.
- ↑ 3.0 3.1 3.2 3.3 Mulinax, R. L.; Okin, G. S.; Coombe, R. D. (1995). "Gas Phase Synthesis, Structure, and Dissociation of Boron Triazide" (in en). The Journal of Physical Chemistry 99 (17): 6294–6300. doi:10.1021/j100017a007. ISSN 0022-3654. https://pubs.acs.org/doi/abs/10.1021/j100017a007.
- ↑ 4.0 4.1 Al-Jihad, Ismail A.; Liu, Bing; Linnen, Christopher J.; Gilbert, Julanna V. (1998). "Generation of NNBN via Photolysis of B(N 3 ) 3 in Low-Temperature Argon Matrices: IR Spectra and ab Initio Calculations" (in en). The Journal of Physical Chemistry A 102 (31): 6220–6226. doi:10.1021/jp9812684. ISSN 1089-5639. Bibcode: 1998JPCA..102.6220A. https://pubs.acs.org/doi/10.1021/jp9812684.
- ↑ Travers, Michael J.; Gilbert, Julanna V. (2000). "UV Absorption Spectra of Intermediates Generated via Photolysis of B(N 3 ) 3 , BCl(N 3 ) 2 , and BCl 2 (N 3 ) in Low-Temperature Argon Matrices †" (in en). The Journal of Physical Chemistry A 104 (16): 3780–3785. doi:10.1021/jp993939j. ISSN 1089-5639. Bibcode: 2000JPCA..104.3780T. https://pubs.acs.org/doi/10.1021/jp993939j.
- ↑ Wiberg, Egon; Michaud, Horst (1954-07-01). "Notizen: Zur Kenntnis eines ätherlöslichen Lithiumborazids LiB(N3)4". Zeitschrift für Naturforschung B 9 (7): 499. doi:10.1515/znb-1954-0716. ISSN 1865-7117.
- ↑ Fraenk, Wolfgang; Habereder, Tassilo; Hammerl, Anton; Klapötke, Thomas M.; Krumm, Burkhard; Mayer, Peter; Nöth, Heinrich; Warchhold, Marcus (2001). "Highly Energetic Tetraazidoborate Anion and Boron Triazide Adducts †" (in en). Inorganic Chemistry 40 (6): 1334–1340. doi:10.1021/ic001119b. ISSN 0020-1669. PMID 11300838. https://pubs.acs.org/doi/10.1021/ic001119b.
Further reading
- Fraenk, W.; Klapötke, T. M. (2002). "Recent Developments in the Chemistry of Covalent Main Group Azides.". in Meyer, G.. Inorganic Chemistry Highlights. Wiley-VCH Verlag. pp. 259–265. ISBN 3-527-30265-4.
 |

