Chemistry:Lithium azide
From HandWiki
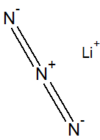
| |
| Error creating thumbnail: Unable to save thumbnail to destination | |
| Names | |
|---|---|
| IUPAC name
lithium azide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| LiN 3 | |
| Molar mass | 48.96 g·mol−1 |
| Melting point | 115 °C (239 °F; 388 K) |
| 36.12 g/100 g (10 °C) 62.07 g/100 g (15.5 °C) 66.41 g/100 g (16 °C)[2] | |
| Solubility | 20.26 g/100 g (16 °C, ethanol)[2] |
| Hazards | |
| Safety data sheet | External SDS |
| GHS pictograms | 
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated.
Preparation
It can be prepared by metathesis reaction between sodium azide and lithium nitrate or lithium sulfate solutions:
- NaN
3 + LiNO
3 → LiN
3 + NaNO
3 - 2 NaN
3 + Li
2SO
4 → 2 LiN
3 + Na
2SO
4[3]
It can also be prepared by reacting lithium sulfate with barium azide.
- Ba(N
3)
2 + Li
2SO
4 → 2 LiN
3 + BaSO
4[2]
References
- ↑ Pringle, G. E.; Noakes, D. E. (February 1968). "The crystal structures of lithium, sodium and strontium azides". Acta Crystallogr. B 24 (2): 262–269. doi:10.1107/S0567740868002062.
- ↑ 2.0 2.1 2.2 Hála, Jiri. "IUPAC-NIST Solubility Data Series. 79. Alkali and Alkaline Earth Metal Pseudohalides". http://nist.gov/data/PDFfiles/jpcrd643.pdf. Retrieved 31 January 2018.
- ↑ "Λ » LambdaSyn – Darstellung von Lithiumazid". http://www.lambdasyn.org/synfiles/lithiumazid.htm.
 |
