Chemistry:Cerium stearate
| |
| Names | |
|---|---|
| Other names
cerium(3+) octadecanoate, cerous stearate, cerium tristearate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C54H105CeO6 | |
| Molar mass | 989.69 |
| Appearance | white powder |
| Density | g/cm3 |
| Melting point | 120 °C (248 °F; 393 K) |
| insoluble | |
| Hazards | |
| P262, P280, P305+351+338, P304+340, P403+233, PP501Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
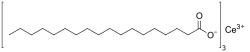
Cerium stearate is a metal-organic compound, a salt of cerium and stearic acid with the chemical formula C54H105CeO6.[2][3] The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.[4]
Synthesis
Cerium stearate is synthesized from the reaction of cerium oxide with stearic acid in an inert atmosphere at temperatures between 100 and 200 °C.[5] It can also be obtained by the reaction of cerium nitrate and potassium stearate.[6]
Physical properties
The compound forms a white powder which is insoluble in water.[citation needed]
Uses
The compound is used in a variety of industrial and laboratory applications: as a lubricant, antioxidant, and antifoaming agent. Other uses include as a catalyst in the synthesis of polymers and as a stabilizer in the production of plastics.[5]
References
- ↑ "NCATS Inxight Drugs — CEROUS STEARATE" (in en). drugs.ncats.io. https://drugs.ncats.io/drug/4S541N00JC.
- ↑ "Cerium Stearate-BEYONDCHEM". beyondchem.com. https://www.beyondchem.com/productinfo/428148.html.
- ↑ "Cerium Stearate" (in en). American Elements. https://www.americanelements.com/cerium-stearate-10119-53-6.
- ↑ "CAS 14536-00-6 Cerium(3+)stearate - Alfa Chemistry". alfa-chemistry.com. https://www.alfa-chemistry.com/cas_14536-00-6.htm.
- ↑ 5.0 5.1 "Buy Cerium stearate - 10119-53-6 | BenchChem". .benchchem.com. https://www.benchchem.com/product/b159660.
- ↑ Marques, Eduardo F.; Burrows, Hugh D.; Miguel, Maria da Graca (1 January 1998). "The structure and thermal behaviour of some long chain cerium(III) carboxylates" (in en). Journal of the Chemical Society, Faraday Transactions 94 (12): 1729–1736. doi:10.1039/A800326B. ISSN 1364-5455. https://pubs.rsc.org/en/content/articlelanding/1998/FT/a800326b. Retrieved 7 March 2023.
 |

