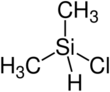
Chemistry:Chlorodimethylsilane
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chlorodi(methyl)silane | |||
| Other names
Dimethylchlorosilane, DMCS
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 2924 | ||
| |||
| |||
| Properties | |||
| (CH3)2SiHCl | |||
| Molar mass | 94.62 g/mol | ||
| Density | 0.852 g/mL, 25 °C | ||
| Melting point | −111 °C (−168 °F; 162 K) | ||
| Boiling point | 34.7 °C (94.5 °F; 307.8 K) | ||
| Hazards | |||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H224, H261, H314, H331 | |||
| P210, P231+232, P233, P240, P241, P242, P243, P260, P261, P264, P271, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P311, P321, P363, P370+378, P402+404, P403+233, P403+235, P405 | |||
| Related compounds | |||
Related compounds
|
Dichloromethylsilane, Trichlorosilane, Trimethylsilane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorodimethylsilane, also called dimethylchlorosilane and abbreviated DMCS, is a chemical compound with the formula (CH3)2SiHCl. It is a silane, with a silicon atom bonded to two methyl groups, a chlorine atom, and a hydrogen atom.
Its structure, including bond lengths and bond angles, has been determined using Fourier transform microwave spectroscopy.[2]
References
- ↑ Sigma-Aldrich Handbook of Fine Chemicals 2007, page 654.
- ↑ Kawashima, Y. (May 2001). "The rotational spectrum of chlorodimethylsilane using Fourier transform microwave spectroscopy". Journal of Molecular Structure 563-564 (1–3): 227–230. doi:10.1016/S0022-2860(00)00879-6. Bibcode: 2001JMoSt.563..227K.
 |