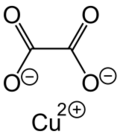
Chemistry:Copper oxalate
| |
| Names | |
|---|---|
| Other names
Copper (II) oxalate, cupric oxalate, copper(2+) ethanedioate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| CuC2O4 | |
| Molar mass | 151.56 |
| Appearance | blue-white solid (as a hemihydrate) |
| Melting point | 310 °C (590 °F; 583 K) |
| insoluble | |
Solubility product (Ksp)
|
4.43×10−10[1] |
| Hazards | |
| GHS pictograms |  [2] [2]
|
| GHS Signal word | Warning |
| H302+312Script error: No such module "Preview warning".Category:GHS errors, H302, H312 | |
| P264, P270, P280, P301+312, P302+352, P312, P322, P330, P363, P501 | |
| Related compounds | |
Related compounds
|
Calcium oxalate Sodium oxalate Magnesium oxalate Strontium oxalate Barium oxalate Iron(II) oxalate Iron(III) oxalate Lithium oxalate Praseodymium oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Copper oxalate is an inorganic compound, a salt of copper metal and oxalic acid with the chemical formula CuC2O4.[3] The compound is practically insoluble in water, alcohol, ether, and acetic acid but soluble in ammonium hydroxide.[4] Copper oxalate forms a hydrate, which forms acid-blue crystals.
Synthesis
Copper oxalate can be produced by precipitation from a mixture of a copper (II) salt and a sodium oxalate solution or by reacting copper sulfate with oxalic acid.[5]
- [math]\displaystyle{ \mathsf{CuSO_4 + H_2C_2O_4 + H_2O \ \xrightarrow{}\ CuC_2O_4\cdot H_2O\downarrow + H_2SO_4 } }[/math]
Properties
As a hemihydrate, copper oxalate is a blue-white solid that is practically insoluble in water. At 200 °C, it loses its water of crystallization.
The compound also forms complex salts with alkali metal oxalates and ammonium oxalate:
- [math]\displaystyle{ \mathsf{CuC_2O_4 + K_2C_2O_4 + 2 H_2O \ \xrightarrow{}\ K_2[Cu(C_2O_4)_2]\cdot 2 H_2O } }[/math]
Uses
Copper oxalate is used as a catalyst for organic reactions, as a stabilizer for acetylated polyformaldehyde[6] and in seed treatment (to repel birds and rodents).[citation needed]
See also
References
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- ↑ "Copper oxalate - Substance Information - ECHA". European Chemical Agency. https://echa.europa.eu/substance-information/-/substanceinfo/100.011.283.
- ↑ Royappa, A. Timothy; Royappa, Andrew D.; Moral, Raphael F.; Rheingold, Arnold L.; Papoular, Robert J.; Blum, Deke M.; Duong, Tien Q.; Stepherson, Jacob R. et al. (November 2016). "Copper(I) oxalate complexes: Synthesis, structures and surprises". Polyhedron 119: 563–574. doi:10.1016/j.poly.2016.09.043.
- ↑ "Hazardous Substances Data Bank (HSDB) : 265" (in en). National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/265.
- ↑ Gooch, Frank Austin (1909). The precipitation of copper oxalate in analysis. p. 448. OCLC 890741677.
- ↑ Richardson, H. Wayne (1997). Handbook of Copper Compounds and Applications. CRC Press. p. 84. ISBN 978-0-8247-8998-5. https://books.google.com/books?id=Zk0z22smWUoC&pg=PA84.
 |


