Chemistry:Copper hydride

| |
| Names | |
|---|---|
| IUPAC name
Copper hydride
| |
| Other names
Copper(I) hydride
Cuprous hydride Hydridocopper(I) Cuprane poly[cuprane(1)] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| CuH | |
| Molar mass | 64.554 g·mol−1 |
| Melting point | 100 °C (212 °F; 373 K)[1] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H228, H315, H319, H335 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[2] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[2] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Copper hydride is an inorganic compound with the chemical formula CuHn where n ~ 0.95.[3] It is a red solid, rarely isolated as a pure composition, that decomposes to the elements.[4] Copper hydride is mainly produced as a reducing agent in organic synthesis and as a precursor to various catalysts.[5]
History
In 1844, the French chemist Adolphe Wurtz synthesised copper hydride for the first time.[6] He reduced an aqueous solution of copper(II) sulfate with hypophosphorous acid (H3PO2). In 2011, Panitat Hasin and Yiying Wu were the first to synthesise a metal hydride (copper hydride) using the technique of sonication.[7] Copper hydride has the distinction of being the first metal hydride discovered. In 2013, it was established by Donnerer et al. that, at least up to fifty gigapascals, copper hydride cannot be synthesised by pressure alone. However, they were successful in synthesising several copper-hydrogen alloys under pressure.[5]
Chemical properties
Structure
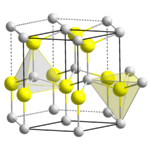
In copper hydride, elements adopt the Wurtzite crystal structure[8][9] (polymeric), being connected by covalent bonds.[1]
The CuH consists of a core of CuH with a shell of water and this may be largely replaced by ethanol. This offers the possibility of modifying the properties of CuH produced by aqueous routes.[10] While all methods for the synthesis of CuH result in the same bulk product, the synthetic path taken engenders differing surface properties. The different behaviors of CuH obtained by aqueous and nonaqueous routes can be ascribed to a combination of very different particle size and dissimilar surface termination, namely, bonded hydroxyls for the aqueous routes and a coordinated donor for the nonaqueous routes.[11]
Chemical reactions
CuH generally behaves as a source of H–. For instance, Wurtz reported the double displacement reaction of CuH with hydrochloric acid:[12]
- CuH + HCl → CuCl +H2
When not cooled below −5 °C (23 °F), copper hydride decomposes, to produce hydrogen gas and a mixture containing elemental copper:
- 2 CuH → xCu•(2-x)CuH + ½x H2 (0 < x < 2)
Solid copper hydride is the irreversible autopolymerisation product of the molecular form, and the molecular form cannot be isolated in concentration.
Production
Copper does not react with hydrogen even on heating,[13] thus copper hydrides are made indirectly from copper(I) and copper(II) precursors. Examples include the reduction of copper(II) sulfate with sodium hypophosphite in the presence of sulfuric acid,[1] or more simply with just hypophosphorous acid.[14] Other reducing agents, including classical aluminium hydrides can be used.[15]
- 4 Cu2+ + 6 H3PO2 + 6 H2O → 4 CuH + 6 H3PO3 + 8 H+
The reactions produce a red-colored precipitate of CuH, which is generally impure and slowly decomposes to liberate hydrogen, even at 0 °C.[14]
- 2 CuH → 2 Cu + H2
This slow decomposition also takes place underwater,[16] however there are reports of the material becoming pyrophoric if dried.[17]
A new synthesis method has been published in 2017 by Lousada et al.[18] In this synthesis high purity CuH nanoparticles have been obtained from basic copper carbonate, CuCO3·Cu(OH)2.[18] This method is faster and has a higher chemical yield than the copper sulfate based synthesis and produces nanoparticles of CuH with higher purity and a smaller size distribution. The obtained CuH can easily be converted to conducting thin films of Cu. These films are obtained by spraying the CuH nanoparticles in their synthesis medium into some insulating support. After drying, conducting Cu films protected by a layer of mixed copper oxides are spontaneously formed.
Reductive sonication
Copper hydride is also produced by reductive sonication. In this process, hexaaquacopper(II) and hydrogen(•) react to produce copper hydride and oxonium according to the equation:
- [Cu(H2O)6]2+ + 3 H• → 1/n (CuH)n + 2 [H3O]+ + 4 H2O
Hydrogen(•) is obtained in situ from the homolytic sonication of water. Reductive sonication produces molecular copper hydride as an intermediate.[7]
Applications in Organic Synthesis
This article relies too much on references to primary sources. (July 2021) (Learn how and when to remove this template message) |
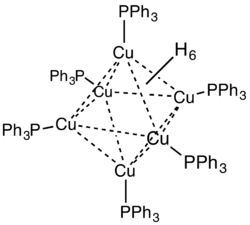
Phosphine- and NHC-copper hydride species have been developed as reagents in organic synthesis, albeit of limited use.[19] Most widely used is [(Ph3P)CuH]6 (Stryker's reagent) for the reduction of α,β-unsaturated carbonyl compounds.[20] H2 (at least 80 psi) and hydrosilanes can be used as the terminal reductant, allowing a catalytic amount of [(Ph3P)CuH]6 to be used for conjugate reduction reactions.[21][22]
Chiral phosphine-copper complexes catalyze hydrosilation of ketones and esters with low enantioselectivities.[23] An enantioselective (80 to 92% ee) reduction of prochiral α,β-unsaturated esters uses Tol-BINAP complexes of copper in the presence of PMHS as the reductant.[24] Subsequently, conditions have been developed for the CuH-catalyzed hydrosilylation of ketones[25] and imines[26] proceeding with excellent levels of chemo- and enantioselectivity.
The reactivity of LnCuH species with weakly activated (e.g. styrenes, dienes) and unactivated alkenes (e.g. α-olefins) and alkynes has been recognized[27] and has served as the basis for several copper-catalyzed formal hydrofunctionalization reactions.[28][29][30]
"Hydridocopper"
The diatomic species CuH is a gas that has attracted the attention of spectroscopists. It polymerises upon being condensed. A well-known oligomer is octahedro-hexacuprane(6), occurring in Stryker's reagent. Hydridocopper has acidic behavior for the same reason as normal copper hydride. However, it does not form stable aqueous solutions, due in part to its autopolymerisation, and its tendency to be oxidised by water. Copper hydride reversibly precipitates from pyridine solution, as an amorphous solid. However, repeated dissolution affords the regular crystalline form, which is insoluble. Under standard conditions, molecular copper hydride autopolymerises to form the crystalline form, including under aqueous conditions, hence the aqueous production method devised by Wurtz.
Production
Molecular copper hydride can be formed by reducing copper iodide with lithium aluminium hydride in ether and pyridine.[31] 4CuI + LiAlH4 → CuH + LiI + AlI3 This was discovered by E Wiberg and W Henle in 1952.[32] The solution of this CuH in the pyridine is typically dark red to dark orange.[31] A precipitate is formed if ether is added to this solution.[31] This will redissolve in pyridine. Impurities of the reaction products remain in the product.[31] In this study, it was found that the solidified diatomic substance is distinct from the Wurtzite structure. The Wurtzite substance was insoluble and was decomposed by lithium iodide, but not the solidified diatomic species. Moreover, while the Wurtzite substance's decomposition is strongly base catalysed, whereas the solidified diatomic species is not strongly affected at all. Dilts distinguishes between the two copper hydrides as the 'insoluble-' and 'soluble copper hydrides'. The soluble hydride is susceptible to pyrolysis under vacuum and proceeds to completion under 100 °C.
Amorphous copper hydride is also produced by anhydrous reduction. In this process copper(I) and tetrahydroaluminate react to produce molecular copper hydride and triiodoaluminium adducts. The molecular copper hydride is precipitated into amorphous copper hydride with the addition of diethyl ether. Amorphous copper hydride is converted into the Wurtz phase by annealing, accompanied by some decomposition.[31]
History
Hydridocopper was discovered in the vibration-rotation emission of a hollow-cathode lamp in 2000 by Bernath, who detected it at the University of Waterloo. It was first detected as a contaminant while attempting to generate NeH+ using the hollow-cathode lamp.[33][34] Molecular copper hydride has the distinction of being the first metal hydride to be detected in this way. (1,0) (2,0) and (2,1) vibrational bands were observed along with line splitting due to the presence of two copper isotopes, 63Cu and 65Cu.[35][36]
The A1Σ+-X1Σ+ absorption lines from CuH have been claimed to have been observed in sunspots and in the star 19 Piscium.[37][38]
In vapour experiments, it was found that copper hydride is produced from the elements upon exposure to 310 nanometre radiation.[4]
- Cu + H2 ↔ CuH + H•
However, this proved to be unviable as a production method as the reaction is difficult to control. The activation barrier for the reverse reaction is virtually non-existent, which allows it to readily proceed even at 20 Kelvin.
Other copper hydrides
- A binary dihydride (CuH2) also exists, in the form of an unstable reactive intermediate in the reduction of copper hydride by atomic hydrogen.
- Ligated copper hydrides (Cu(I)H) can participate in hydrocupration reactions.
References
- ↑ 1.0 1.1 1.2 Fitzsimons, Nuala P.; Jones, William; Herley, Patrick J. (1 January 1995). "Studies of copper hydride. Part 1.—Synthesis and solid-state stability". Journal of the Chemical Society, Faraday Transactions 91 (4): 713–718. doi:10.1039/FT9959100713.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0150.html.
- ↑ Jordan, Abraham J.; Lalic, Gojko; Sadighi, Joseph P. (2016-07-25). "Coinage Metal Hydrides: Synthesis, Characterization, and Reactivity". Chemical Reviews 116 (15): 8318–8372. doi:10.1021/acs.chemrev.6b00366. ISSN 0009-2665. PMID 27454444.
- ↑ 4.0 4.1 Aldridge, Simon; Downs, Anthony J. (2001). "Hydrides of the Main-Group Metals: New Variations on an Old Theme". Chem. Rev. 101 (11): 3305–3366. doi:10.1021/cr960151d. PMID 11840988.
- ↑ 5.0 5.1 Donnerer, Christian; Scheler, Thomas; Gregoryanz, Eugene (4 April 2013). "High-pressure synthesis of noble metal hydrides". The Journal of Chemical Physics 138 (13): 134507. doi:10.1063/1.4798640. PMID 23574244. Bibcode: 2013JChPh.138m4507D. http://jcp.aip.org/resource/1/jcpsa6/v138/i13/p134507_s1. Retrieved 20 June 2013.
- ↑ Wurtz, A. (1844) "Sur l'hydrure de cuivre" (On copper hydride), Comptes rendus, 18 : 702–704.
- ↑ 7.0 7.1 Hasin, Panitat; Wu, Yiying (1 January 2012). "Sonochemical synthesis of copper hydride (CuH)". Chemical Communications 48 (9): 1302–1304. doi:10.1039/C2CC15741A. PMID 22179137.
- ↑ Goedkoop, J. A.; Andresen, A. F. (1955). "The crystal structure of copper hydride". Acta Crystallographica 8 (2): 118–119. doi:10.1107/S0365110X55000480. Bibcode: 1955AcCry...8..118G.
- ↑ Müller, Heinz; Bradley, Albert James (1926). "CCXVII.—Copper hydride and its crystal structure". Journal of the Chemical Society (Resumed) 129: 1669–1673. doi:10.1039/JR9262901669.
- ↑ Bennett, Elliot L; Thomas Wilson; Patrick J Murphy; Keith Refson; Alex C Hannon; Silvia Imberti; Samantha K Callear; Gregory A Chass et al. (2015). "Structure and spectroscopy of CuH prepared via borohydride reduction". Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials 71 (6): 608–612. doi:10.1107/S2052520615015176. PMID 26634717. Bibcode: 2015AcCrB..71..608B.
- ↑ Bennett, Elliot L; Thomas Wilson; Patrick J Murphy; Keith Refson; Alex C Hannon; Silvia Imberti; Samantha K Callear; Gregory A Chass et al. (2015). "How the Surface Structure Determines the Properties of CuH". Inorganic Chemistry 54 (5): 2213–2220. doi:10.1021/ic5027009. PMID 25671787.
- ↑ Rocke, Alan J. (2001). Nationalizing Science: Adolphe Wurtz and the Battle for French Chemistry. Cambridge, MA: MIT Press. pp. 121–122. ISBN 978-0-262-26429-7.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 14.0 14.1 Burtovyy, R.; Utzig, E.; Tkacz, M. (2000). "Studies of the thermal decomposition of copper hydride". Thermochimica Acta 363 (1–2): 157–163. doi:10.1016/S0040-6031(00)00594-3. Bibcode: 2000TcAc..363..157B.
- ↑ Brauer, Georg (1963). Handbook of Preparative Inorganic Chemistry. 2 (2nd ed.). New York: Academic Press. p. 1004. ISBN 978-0-323-16129-9. https://books.google.com/books?id=Pef47TK5NfkC.
- ↑ Warf, James C.; Feitknecht, W. (1950). "Zur Kenntnis des Kupferhydrids, insbesondere der Kinetik des Zerfalls". Helvetica Chimica Acta 33 (3): 613–639. doi:10.1002/hlca.19500330327. Bibcode: 1950HChAc..33..613W.
- ↑ Goedkoop, J. A.; Andresen, A. F. (1955). "The crystal structure of copper hydride". Acta Crystallogr 8 (2): 118–119. doi:10.1107/S0365110X55000480. Bibcode: 1955AcCry...8..118G.
- ↑ 18.0 18.1 Lousada, Cláudio M.; Fernandes, Ricardo M. F.; Tarakina, Nadezda V.; Soroka, Inna L. (2017). "Synthesis of copper hydride (CuH) from CuCO3·Cu(OH)2 – a path to electrically conductive thin films of Cu" (in en). Dalton Transactions 46 (20): 6533–6543. doi:10.1039/C7DT00511C. ISSN 1477-9226. PMID 28379275.
- ↑ Whitesides, George M.; San Filippo, Joseph; Stredronsky, Erwin R.; Casey, Charles P. (1969-11-01). "Reaction of copper(I) hydride with organocopper(I) compounds". Journal of the American Chemical Society 91 (23): 6542–6544. doi:10.1021/ja01051a093. ISSN 0002-7863. Bibcode: 1969JAChS..91.6542W.
- ↑ John F. Daeuble and Jeffrey M. Stryker "Hexa-μ-hydrohexakis(triphenylphosphine)hexacopper" eEROS Encyclopedia of Reagents for Organic Synthesis, 2001. doi:10.1002/047084289X.rh011m
- ↑ Mahoney, Wayne S.; Stryker, Jeffrey M. (1989-11-01). "Hydride-mediated homogeneous catalysis. Catalytic reduction of α,β-unsaturated ketones using [(Ph3P)CuH]6 and H2". Journal of the American Chemical Society 111 (24): 8818–8823. doi:10.1021/ja00206a008. ISSN 0002-7863.
- ↑ Mori, Atsunori; Fujita, Akinori (1997-01-01). "Copper(I) salt mediated 1,4-reduction of α,β-unsaturated ketones using hydrosilanes" (in en). Chemical Communications (22): 2159–2160. doi:10.1039/a706032g. ISSN 1364-548X. http://www.lib.kobe-u.ac.jp/repository/90000454.pdf. Retrieved 2019-12-19.
- ↑ Brunner, Henri; Miehling, Wolfgang (1984-10-23). "Asymmetrische katalysen". Journal of Organometallic Chemistry 275 (2): c17–c21. doi:10.1016/0022-328X(84)85066-4.
- ↑ Appella, Daniel H.; Moritani, Yasunori; Shintani, Ryo; Ferreira, Eric M.; Buchwald, Stephen L. (1999-10-01). "Asymmetric Conjugate Reduction of α,β-Unsaturated Esters Using a Chiral Phosphine−Copper Catalyst". Journal of the American Chemical Society 121 (40): 9473–9474. doi:10.1021/ja992366l. ISSN 0002-7863. Bibcode: 1999JAChS.121.9473A.
- ↑ Lipshutz, Bruce H.; Noson, Kevin; Chrisman, Will; Lower, Asher (2003-07-01). "Asymmetric Hydrosilylation of Aryl Ketones Catalyzed by Copper Hydride Complexed by Nonracemic Biphenyl Bis-phosphine Ligands". Journal of the American Chemical Society 125 (29): 8779–8789. doi:10.1021/ja021391f. ISSN 0002-7863. PMID 12862472. Bibcode: 2003JAChS.125.8779L.
- ↑ Lipshutz, Bruce H.; Shimizu, Hideo (2004-04-19). "Copper(I)-Catalyzed Asymmetric Hydrosilylations of Imines at Ambient Temperatures" (in en). Angewandte Chemie International Edition 43 (17): 2228–2230. doi:10.1002/anie.200353294. ISSN 1521-3773. PMID 15108129. Bibcode: 2004ACIE...43.2228L.
- ↑ Noh, Dongwan; Chea, Heesung; Ju, Junghwan; Yun, Jaesook (2009-08-03). "Highly Regio- and Enantioselective Copper-Catalyzed Hydroboration of Styrenes" (in en). Angewandte Chemie International Edition 48 (33): 6062–6064. doi:10.1002/anie.200902015. ISSN 1521-3773. PMID 19591178.
- ↑ Miki, Yuya; Hirano, Koji; Satoh, Tetsuya; Miura, Masahiro (2013-10-04). "Copper-Catalyzed Intermolecular Regioselective Hydroamination of Styrenes with Polymethylhydrosiloxane and Hydroxylamines" (in en). Angewandte Chemie International Edition 52 (41): 10830–10834. doi:10.1002/anie.201304365. ISSN 1521-3773. PMID 24038866. Bibcode: 2013ACIE...5210830M.
- ↑ Zhu, Shaolin; Niljianskul, Nootaree; Buchwald, Stephen L. (2013-10-23). "Enantio- and Regioselective CuH-Catalyzed Hydroamination of Alkenes". Journal of the American Chemical Society 135 (42): 15746–15749. doi:10.1021/ja4092819. ISSN 0002-7863. PMID 24106781. Bibcode: 2013JAChS.13515746Z.
- ↑ Uehling, Mycah R.; Rucker, Richard P.; Lalic, Gojko (2014-06-18). "Catalytic Anti-Markovnikov Hydrobromination of Alkynes". Journal of the American Chemical Society 136 (24): 8799–8803. doi:10.1021/ja503944n. ISSN 0002-7863. PMID 24896663. Bibcode: 2014JAChS.136.8799U.
- ↑ 31.0 31.1 31.2 31.3 31.4 Dilts, J. A.; D. F. Shriver (1968). "Nature of soluble copper(I) hydride". Journal of the American Chemical Society 90 (21): 5769–5772. doi:10.1021/ja01023a020. ISSN 0002-7863. Bibcode: 1968JAChS..90.5769D.
- ↑ E Wiberg; W Henle (1952). "Über die Dämpfung der elektromagnetischen Eigenschwingungen des Systems Erde — Luft — Ionosphäre". Zeitschrift für Naturforschung A 7 (3–4): 250. doi:10.1515/zna-1952-3-404. Bibcode: 1952ZNatA...7..250S.
- ↑ Bernath, P. F. (2000). "6 Infrared emission spectroscopy". Annual Reports on the Progress of Chemistry, Section C 96 (1): 202. doi:10.1039/B001200I. ISSN 0260-1826. https://molspect.chemistry.ohio-state.edu/institute/bernath4-IRemissionreview.pdf. Retrieved 2013-02-23.
- ↑ Ram, R.S.; P.F. Bernath; J.W. Brault (1985). "Fourier transform emission spectroscopy of NeH+". Journal of Molecular Spectroscopy 113 (2): 451–457. doi:10.1016/0022-2852(85)90281-4. ISSN 0022-2852. Bibcode: 1985JMoSp.113..451R.
- ↑ Ram, R. S.; P.F. Bernath; J.W. Brault (1985). Cameron, David G; Grasselli, Jeannette G. eds. "Infrared Fourier Transform Emission Spectroscopy of CuH and NeH+". Proc. SPIE. Fourier and Computerized Infrared Spectroscopy 553: 774–775. doi:10.1117/12.970862. Bibcode: 1985SPIE..553..374R.
- ↑ Seto, Jenning Y.; Zulfikar Morbi; Frank Charron; Sang K. Lee; Peter F. Bernath; Robert J. Le Roy (1999). "Vibration-rotation emission spectra and combined isotopomer analyses for the coinage metal hydrides: CuH & CuD, AgH & AgD, and AuH & AuD". The Journal of Chemical Physics 110 (24): 11756. doi:10.1063/1.479120. ISSN 0021-9606. Bibcode: 1999JChPh.11011756S.
- ↑ Wojslaw, Robert S.; Benjamin F. Peery (May 1976). "Identification of Novel Molecules in the Spectrum of 19 Piscium". The Astrophysical Journal Supplement 31: 75–92. doi:10.1086/190375. Bibcode: 1976ApJS...31...75W.
- ↑ Fernando, W. T. M. L.; L. C. O'Brien; P. F. Bernath (1990). "Fourier Transform Emission Spectroscopy of the A1Σ+-X1Σ+ Transition of CuD". Journal of Molecular Spectroscopy 139 (2): 461–464. doi:10.1016/0022-2852(90)90084-4. ISSN 0022-2852. Bibcode: 1990JMoSp.139..461F. http://bernath.uwaterloo.ca/media/70.pdf. Retrieved 2013-02-20.
 |







