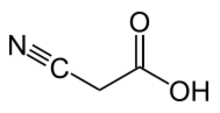
Chemistry:Cyanoacetic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Cyanoacetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 506325 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1759 |
| |
| |
| Properties | |
| C3H3NO2 | |
| Molar mass | 85.06 g/mol |
| Appearance | colorless solid |
| Density | 1.287 g/cm3 |
| Melting point | 69-70 °C |
| Boiling point | 108 °C (15 mm Hg) |
| 1000 g/L (20 °C) in water | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H314, H332 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P330, P363, P405, P501 | |
| Flash point | 107 °C (225 °F; 380 K) |
| Related compounds | |
Related
|
Ethyl cyanoacetate Cyanoacetamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyanoacetic acid is an organic compound. It is a white, hygroscopic solid. The compound contains two functional groups, a nitrile (−C≡N) and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives.[1]
Preparation and reactions
Cyanoacetic acid is prepared by treatment of chloroacetate salts with sodium cyanide followed by acidification.[1][2] Electrosynthesis by cathodic reduction of carbon dioxide and anodic oxidation of acetonitrile also affords cyanoacetic acid.[3]
Cyanoacetic acid is used to do cyanoacetylation, first convenient method described by J. Slätt.[4]
It is about 1000x more acidic than acetic acid, with a pKa of 2.5. Upon heating at 160 °C, it undergoes decarboxylation to give acetonitrile:
- C3H3NO2 → C2H3N + CO2
Applications
The largest scale reaction is its esterification to give the corresponding ester ethyl cyanoacetate, which is then transformed to ethyl cyanoacrylate used as superglue, via reaction with formaldehyde. As of 2007, more than 10,000 tons of cyanoacetic acid were produced annually.
Cyanoacetic acid is a versatile intermediate in the preparation of chemicals. it is a precursor to synthetic caffeine via the intermediacy of theophylline. It is a building block for many drugs, including dextromethorphan, amiloride, sulfadimethoxine, and allopurinol,[1] and also for Peldesine.
Safety
The LD50 (oral, rats) is 1.5 g/kg.[1]
References
- ↑ 1.0 1.1 1.2 1.3 Harald Strittmatter, Stefan Hildbrand and Peter Pollak Malonic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a16_063.pub2
- ↑ Inglis, J. K. H. (1928). "Ethyl Cyanoacetate". Organic Syntheses 8: 74. doi:10.15227/orgsyn.008.0074.
- ↑ Barba, Fructuoso; Batanero, Belen (2004). "Paired Electrosynthesis of Cyanoacetic Acid". The Journal of Organic Chemistry 69 (7): 2423–2426. doi:10.1021/jo0358473. PMID 15049640.
- ↑ Bergman, Jan; Romero, Ivan; Slätt, Johnny (2004). "Cyanoacetylation of indoles, pyrroles and aromatic amines with the combination cyanoacetic acid and acetic anhydride". Synthesis 2004 (16): 2760–2765. doi:10.1055/s-2004-831164. http://urn.kb.se/resolve?urn=urn:nbn:se:kth:diva-92000.
 |

