Biology:Theophylline
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Theolair, Slo-Bid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681006 |
| Pregnancy category |
|
| Routes of administration | oral, IV, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (oral) |
| Protein binding | 40% (primarily to albumin) |
| Metabolism | Hepatic to 1-methyluric acid |
| Elimination half-life | 5–8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
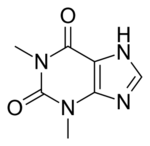
| Formula | C7H8N4O2 |
| Molar mass | 180.167 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Theophylline, also known as 1,3-dimethylxanthine, is a drug that inhibits phosphodiesterase and blocks adenosine receptors.[1] It is used to treat chronic obstructive pulmonary disease (COPD) and asthma.[2] Its pharmacology is similar to other methylxanthine drugs (e.g., theobromine and caffeine).[1] Trace amounts of theophylline are naturally present in tea, coffee, chocolate, yerba maté, guarana, and kola nut.[1][3]
The name 'theophylline' derives from "Thea"—the former genus name for tea + Legacy Greek φύλλον (phúllon, “leaf”) + -ine.
Medical uses
The main actions of theophylline involve:[2]
- relaxing bronchial smooth muscle
- increasing heart muscle contractility and efficiency (positive inotrope)
- increasing heart rate (positive chronotropic)
- increasing blood pressure
- increasing renal blood flow
- anti-inflammatory effects
- central nervous system stimulatory effect, mainly on the medullary respiratory center[4]
The main therapeutic uses of theophylline are for treating:[2]
- Chronic obstructive pulmonary disease (COPD)[5]
- Asthma
- infant apnea[6]
- Blocks the action of adenosine; an inhibitory neurotransmitter that induces sleep, contracts the smooth muscles and relaxes the cardiac muscle.
- Treatment of post-dural puncture headache.[7][8]
Performance enhancement in sports
Theophylline and other methylxanthines are often used for their performance-enhancing effects in sports, as these drugs increase alertness, bronchodilation, and increase the rate and force of heart contraction.[9] There is conflicting information about the value of theophylline and other methylxanthines as prophylaxis against exercise-induced asthma.[10]
Adverse effects
The use of theophylline is complicated by its interaction with various drugs and by the fact that it has a narrow therapeutic window (<20 mcg/mL).[2] Its use must be monitored by direct measurement of serum theophylline levels to avoid toxicity. It can also cause nausea, diarrhea, increase in heart rate, abnormal heart rhythms, and CNS excitation (headaches, insomnia, irritability, dizziness and lightheadedness).[2][11] Seizures can also occur in severe cases of toxicity, and are considered to be a neurological emergency.[2]
Its toxicity is increased by erythromycin, cimetidine, and fluoroquinolones, such as ciprofloxacin. Some lipid-based formulations of theophylline can result in toxic theophylline levels when taken with fatty meals, an effect called dose dumping, but this does not occur with most formulations of theophylline.[12] Theophylline toxicity can be treated with beta blockers. In addition to seizures, tachyarrhythmias are a major concern.[13] Theophylline should not be used in combination with the SSRI fluvoxamine.[14][15]
Spectroscopy
UV-visible spectroscopy
Theophylline is soluble in 0.1N NaOH and absorbs maximally at 277 nm with an extinction coefficient of 10,200 (cm−1 M−1).[16]
Proton nuclear magnetic resonance spectroscopy (1H-NMR)
The characteristic signals, distinguishing theophylline from related methylxanthines, are approximately 3.23δ and 3.41δ, corresponding to the unique methylation possessed by theophylline. The remaining proton signal, at 8.01δ, corresponds to the proton on the imidazole ring, not transferred between the nitrogen. The transferred proton between the nitrogen is a variable proton and only exhibits a signal under certain conditions.[17]
Carbon nuclear magnetic resonance spectroscopy (13C-NMR)
The unique methylation of theophylline corresponds to the following signals: 27.7δ and 29.9δ. The remaining signals correspond to carbons characteristic of the xanthine backbone.[18]
Natural occurrences
Theophylline is naturally found in cocoa beans. Amounts as high as 3.7 mg/g have been reported in Criollo cocoa beans.[19]
Trace amounts of theophylline are also found in brewed tea, although brewed tea provides only about 1 mg/L,[20] which is significantly less than a therapeutic dose.
Trace amounts of theophylline are also found in guarana (Paullinia cupana) and in kola nuts.[21]
Pharmacology
Pharmacodynamics
Like other methylated xanthine derivatives, theophylline is both a
- competitive nonselective phosphodiesterase inhibitor which increases intracellular levels of cAMP and cGMP,[2][22] activates PKA, inhibits TNF-alpha[23][24] and inhibits leukotriene[25] synthesis, and reduces inflammation and innate immunity[25]
- nonselective adenosine receptor antagonist, antagonizing A1, A2, and A3 receptors almost equally, which explains many of its cardiac effects.[2][26] Theophylline activates histone deacetylases.[2]
Pharmacokinetics
Absorption
When theophylline is administered intravenously, bioavailability is 100%.[27]
Distribution
Theophylline is distributed in the extracellular fluid, in the placenta, in the mother's milk and in the central nervous system. The volume of distribution is 0.5 L/kg. The protein binding is 40%.
Metabolism
Theophylline is metabolized extensively in the liver.[2] It undergoes N-demethylation via cytochrome P450 1A2. It is metabolized by parallel first order and Michaelis-Menten pathways. Metabolism may become saturated (non-linear), even within the therapeutic range. Small dose increases may result in disproportionately large increases in serum concentration. Methylation to caffeine is also important in the infant population. Smokers and people with hepatic (liver) impairment metabolize it differently.[2] Cigarette and marihuana smoking induces metabolism of theophylline, increasing the drug's metabolic clearance.[28][29]
Excretion
Theophylline is excreted unchanged in the urine (up to 10%). Clearance of the drug is increased in children (age 1 to 12), teenagers (12 to 16), adult smokers, elderly smokers, as well as in cystic fibrosis, and hyperthyroidism. Clearance of the drug is decreased in these conditions: elderly, acute congestive heart failure, cirrhosis, hypothyroidism and febrile viral illnesses.[2]
The elimination half-life varies: 30 hours for premature neonates, 24 hours for neonates, 3.5 hours for children ages 1 to 9, 8 hours for adult non-smokers, 5 hours for adult smokers, 24 hours for those with hepatic impairment, 12 hours for those with congestive heart failure NYHA class I-II, 24 hours for those with congestive heart failure NYHA class III-IV, 12 hours for the elderly.
History
Theophylline was first extracted from tea leaves and chemically identified around 1888 by the German biologist Albrecht Kossel.[30][31] Seven years later, a chemical synthesis starting with 1,3-dimethyluric acid was described by Emil Fischer and Lorenz Ach.[32] The Traube purine synthesis, an alternative method to synthesize theophylline, was introduced in 1900 by another German scientist, Wilhelm Traube.[33] Theophylline's first clinical use came in 1902 as a diuretic.[34] It took an additional 20 years until it was first reported as an asthma treatment.[35] The drug was prescribed in a syrup up to the 1970s as Theostat 20 and Theostat 80, and by the early 1980s in a tablet form called Quibron.
References
- ↑ 1.0 1.1 1.2 "Theophylline". PubChem, US National Library of Medicine. 26 August 2023. https://pubchem.ncbi.nlm.nih.gov/compound/2153.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 "Theophylline". American Journal of Respiratory and Critical Care Medicine 188 (8): 901–906. October 2013. doi:10.1164/rccm.201302-0388PP. PMID 23672674. https://www.atsjournals.org/doi/10.1164/rccm.201302-0388PP.
- ↑ "Coffee, Tea, Mate, Methylxanthines and Methylglyoxal.". IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (International Agency for Research on Cancer) 51: 391–419. 1991. PMID 2033791. PMC 7681294. https://www.ncbi.nlm.nih.gov/books/NBK507021/#:~:text=Theophylline%20is%20found%20in%20black,used%20principally%20in%20pharmaceutical%20preparations..
- ↑ "Antagonism by theophylline of respiratory inhibition induced by adenosine". Journal of Applied Physiology 59 (5): 1428–1433. November 1985. doi:10.1152/jappl.1985.59.5.1428. PMID 4066573.
- ↑ "Efficacy and side effects of intravenous theophylline in acute asthma: a systematic review and meta-analysis". Drug Design, Development and Therapy 12: 99–120. 10 January 2018. doi:10.2147/DDDT.S156509. PMID 29391776.
- ↑ "Comparative efficacy and safety of caffeine citrate and aminophylline in treating apnea of prematurity: A systematic review and meta-analysis". PLOS ONE 17 (9): e0274882. 19 September 2022. doi:10.1371/journal.pone.0274882. PMID 36121807. Bibcode: 2022PLoSO..1774882M.
- ↑ "The impact of aminophylline on incidence and severity of post-dural puncture headache: A meta-analysis of randomised controlled trials". Anaesthesia, Critical Care & Pain Medicine 40 (4): 100920. August 2021. doi:10.1016/j.accpm.2021.100920. PMID 34186265.
- ↑ "Post-dural puncture headache prevention and treatment with aminophylline or theophylline: a systematic review and meta-analysis". Anesthesia and Pain Medicine 18 (2): 177–189. April 2023. doi:10.17085/apm.22247. PMID 37183286.
- ↑ "Performance-enhancing drugs and the Olympics". Journal of Internal Medicine 291 (2): 181–196. February 2022. doi:10.1111/joim.13431. PMID 35007384.
- ↑ "Exercise-Induced Bronchospasm in Elite Athletes". Cureus 14 (1): e20898. January 2022. doi:10.7759/cureus.20898. PMID 35145802.
- ↑ "Theophylline". MedlinePlus Drug Information. U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a681006.html.
- ↑ "Food-induced "dose-dumping" from a once-a-day theophylline product as a cause of theophylline toxicity". Chest 87 (6): 758–765. June 1985. doi:10.1378/chest.87.6.758. PMID 3996063.
- ↑ "Acute theophylline toxicity and the use of esmolol to reverse cardiovascular instability". Annals of Emergency Medicine 19 (6): 671–673. June 1990. doi:10.1016/s0196-0644(05)82474-6. PMID 1971502.
- ↑ "Fluvoxamine-induced theophylline toxicity". The American Journal of Psychiatry 154 (9): 1317–1318. September 1997. doi:10.1176/ajp.154.9.1317b. PMID 9286199.
- ↑ "Toxic interaction between fluvoxamine and sustained release theophylline in an 11-year-old boy". Drug Safety 6 (6): 460–462. November 1991. doi:10.2165/00002018-199106060-00006. PMID 1793525.
- ↑ "An ultraviolet spectrophotometric method for the determination of theophylline and theobromine in blood and tissues". The Journal of Pharmacology and Experimental Therapeutics 97 (3): 283–291. November 1949. PMID 15392550.
- ↑ "Fragment Discovery for the Design of Nitrogen Heterocycles as Mycobacterium tuberculosis Dihydrofolate Reductase Inhibitors". Archiv der Pharmazie 349 (8): 602–613. August 2016. doi:10.1002/ardp.201600066. PMID 27320965.
- ↑ "Pteridines. Part CXIX. A New Pteridine–Purine Transformation.". Helvetica Chimica Acta 91 (2): 338–353. February 2008. doi:10.1002/hlca.200890039.
- ↑ "Methylxanthine composition and consumption patterns of cocoa and chocolate products and their uses". Caffeine. CRC Press. 1998. p. 171. ISBN 978-0-8493-2647-9. https://books.google.com/books?id=WxmBmvhsoZ8C&pg=PA171. Retrieved 2013-11-10.
- ↑ "TABLE 2a: Concentrations of caffeine, theobromine and theophylline in tea products.". Food Surveillance Information Sheet Number 103. MAFF, Department of Health and the Scottish Executive. http://archive.food.gov.uk/maff/archive/food/infsheet/1997/no103/table2a.htm.
- ↑ "HPLC determination of caffeine and theophylline in Paullinia cupana Kunth (guarana) and Cola spp. samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung 180 (5): 398–401. May 1985. doi:10.1007/BF01027774. PMID 4013524.
- ↑ "Cyclic nucleotide phosphodiesterases". The Journal of Allergy and Clinical Immunology 108 (5): 671–680. November 2001. doi:10.1067/mai.2001.119555. PMID 11692087.
- ↑ "Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition". Clinics 63 (3): 321–328. June 2008. doi:10.1590/S1807-59322008000300006. PMID 18568240.
- ↑ "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". American Journal of Respiratory and Critical Care Medicine 159 (2): 508–511. February 1999. doi:10.1164/ajrccm.159.2.9804085. PMID 9927365.
- ↑ 25.0 25.1 "Leukotrienes: underappreciated mediators of innate immune responses". Journal of Immunology 174 (2): 589–594. January 2005. doi:10.4049/jimmunol.174.2.589. PMID 15634873.
- ↑ "Adenosine receptors: development of selective agonists and antagonists". Progress in Clinical and Biological Research 230 (1): 41–63. 1987. PMID 3588607.
- ↑ The Textbook of Pharmaceutical Medicine (6th ed.). Chichester: Wiley-Blackwell. 2009. ISBN 978-1-4051-8035-1.
- ↑ "Relationship of urinary metabolites of theophylline to serum theophylline levels". Clinical Pharmacology and Therapeutics 19 (3): 375–381. March 1976. doi:10.1002/cpt1976193375. PMID 1261172.
- ↑ "Enhanced biotransformation of theophylline in marihuana and tobacco smokers". Clinical Pharmacology and Therapeutics 24 (4): 405–410. October 1978. doi:10.1002/cpt1978244406. PMID 688731.
- ↑ "Über eine neue Base aus dem Pflanzenreich" (in de). Berichte der Deutschen Chemischen Gesellschaft 21: 2164–2167. 1888. doi:10.1002/cber.188802101422. https://zenodo.org/record/1425531.
- ↑ "Über das Theophyllin, einen neuen Bestandtheil des Thees" (in de). Hoppe-Seyler's Zeitschrift für Physiologische Chemie 13: 298–308. 1889.
- ↑ "Synthese des Caffeins" (in de). Berichte der Deutschen Chemischen Gesellschaft 28 (3): 3139. 1895. doi:10.1002/cber.189502803156.
- ↑ "Der synthetische Aufbau der Harnsäure, des Xanthins, Theobromins, Theophyllins und Caffeïns aus der Cyanessigsäure" (in de). Berichte der Deutschen Chemischen Gesellschaft 33 (3): 3035–3056. 1900. doi:10.1002/cber.19000330352. https://zenodo.org/record/1425990.
- ↑ "Über Theocin (Theophyllin) als Diureticum" (in de). Therapie der Gegenwart 43: 490–493. 1902.
- ↑ "The clinical and pharmacological history of theophylline: first report on the bronchospasmolytic action in man by S. R. Hirsch in Frankfurt (Main) 1922". Clinical Allergy 12 (2): 211–215. March 1982. doi:10.1111/j.1365-2222.1982.tb01641.x. PMID 7042115.
 |
