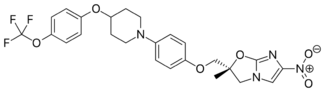
Chemistry:Delamanid
 | |
| Clinical data | |
|---|---|
| Trade names | Deltyba |
| Other names | OPC-67683 |
| AHFS/Drugs.com | UK Drug Information |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ≥99.5% |
| Metabolism | in plasma by albumin, in liver by CYP3A4 (to a lesser extent) |
| Elimination half-life | 30–38 hours |
| Excretion | not excreted in urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H25F3N4O6 |
| Molar mass | 534.492 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Delamanid, sold under the brand name Deltyba, is a medication used to treat tuberculosis.[2] Specifically it is used, along with other antituberculosis medications, for active multidrug-resistant tuberculosis.[2] It is taken by mouth.[2]
Common side effects include headache, dizziness, and nausea.[3] Other side effects include QT prolongation.[2] Delamanid works by blocking the manufacture of mycolic acids thus destabilising the bacterial cell wall.[4] It is in the nitroimidazole class of medications.[5]
Delamanid was approved for medical use in 2014 in Europe, Japan, and South Korea.[6] It is on the World Health Organization's List of Essential Medicines.[7] As of 2016 the Stop TB Partnership had an agreement to get the medication for US$1,700 per six month for use in more than 100 countries.[8]
Medical uses
Delamanid is used, along with other antituberculosis medications, for active multidrug-resistant tuberculosis.[2]
Adverse effects
Common side effects include headache, dizziness, and nausea.[3] Other side effects include QT prolongation.[2] Use in pregnancy has not been extensively studied, but there have been reports of success[9] and it is currently recommended as part of the standard treatment regimen for pregnant women with rifampicin-resistant tuberculosis in South Africa.[10]
Interactions
Delamanid is metabolised by the liver enzyme CYP3A4; therefore strong inducers of this enzyme can reduce its effectiveness.[11]
Mechanism of action
Delamanid is activated in the mycobacterium by deazaflavin-dependent nitroreductase (Ddn), an enzyme which uses dihydro-F420 (reduced form), into nitric oxide and a highly reactive metabolite. This metabolite attacks the synthesis enzyme DprE2, which is important for the synthesis of cell wall arabinogalactan, to which mycolic acid would be attached. This mechanism is shared with pretomanid. Clinical isolates resistant to this drug tend to have mutations in the biosynthetic pathway for Coenzyme F420.[12]
History
In phase II clinical trials, the drug was used in combination with standard treatments, such as four or five of the drugs ethambutol, isoniazid, pyrazinamide, rifampicin, aminoglycoside antibiotics, and quinolones. Healing rates (measured as sputum culture conversion) were significantly better in patients who additionally took delamanid.[13][14]
The European Medicines Agency (EMA) recommended conditional marketing authorization for delamanid in adults with multidrug-resistant pulmonary tuberculosis without other treatment options because of resistance or tolerability. The EMA considered the data show that the benefits of delamanid outweigh the risks, but that additional studies were needed on the long-term effectiveness.[15]
Society and culture
The medication was not readily available globally as of 2015. It was believed that pricing will be similar to bedaquiline, which for six months is approximately US$900 in low income countries, US$3,000 in middle income countries, and US$30,000 in high income countries.[2] As of 2016 the Stop TB Partnership had an agreement to get the medication for US$1,700 per six month.[8]
References
- ↑ "Deltyba (delamanid): Summary of Product Characteristics. 5.2. Pharmacokinetic Properties". Otsuka Novel Products GmbH. p. 10. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002552/WC500166232.pdf.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 The selection and use of essential medicines. Twentieth report of the WHO Expert Committee 2015 (including 19th WHO Model List of Essential Medicines and 5th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. 2015. pp. 30–1. WHO technical report series;994. ISBN 9789241209946.
- ↑ 3.0 3.1 "Drugs Used in Tuberculosis and Leprosy" (in en). Side Effects of Drugs Annual: A Worldwide Yearly Survey of New Data in Adverse Drug Reactions. Elsevier. 2016. p. 284. ISBN 978-0-444-63889-2. https://books.google.com/books?id=km4kDAAAQBAJ&pg=PA284.
- ↑ "Delamanid: a review of its use in patients with multidrug-resistant tuberculosis". Drugs 75 (1): 91–100. January 2015. doi:10.1007/s40265-014-0331-4. PMID 25404020.
- ↑ "Vaccines Against Tuberculosis" (in en). Tropical Diseases: An Overview of Major Diseases Occurring in the Americas. Bentham Science Publishers. 2017. p. 461. doi:10.2174/9781681085876117010022. ISBN 978-1-68108-587-6. https://books.google.com/books?id=uMtFDwAAQBAJ&pg=PA461.
- ↑ (in en) Successful Drug Discovery. John Wiley & Sons. 2016. p. 139. ISBN 978-3-527-34115-3. https://books.google.com/books?id=S70cDQAAQBAJ&pg=PA139.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 8.0 8.1 "Stop TB Partnership | "Stop TB Partnership's Global Drug Facility jumpstarts access to new drugs for MDR-TB with innovative public-private partnerships" (in en). http://www.stoptb.org/news/stories/2016/ns16_005.asp.
- ↑ "Outcomes of Children Born to Pregnant Women With Drug-resistant Tuberculosis Treated With Novel Drugs in Khayelitsha, South Africa: A Report of Five Patients". The Pediatric Infectious Disease Journal 40 (5): e191–e192. May 2021. doi:10.1097/INF.0000000000003069. PMID 33847295.
- ↑ "Clinical Management of Rifampicin-Resistant Tuberculosis: Updated Clinical Reference Guide". September 2023. https://www.health.gov.za/wp-content/uploads/2023/10/Updated-RR-TB-Clinical-Guidelines-September-2023.pdf.
- ↑ "Delamanid: Neuer Wirkstoff gegen multiresistente TB" (in de). Pharmazeutische Zeitung. 9 May 2014. http://www.pharmazeutische-zeitung.de/index.php?id=52126.
- ↑ "DprE2 is a molecular target of the anti-tubercular nitroimidazole compounds pretomanid and delamanid". Nature Communications 14 (1): 3828. June 2023. doi:10.1038/s41467-023-39300-z. PMID 37380634. Bibcode: 2023NatCo..14.3828A.
- ↑ "Neue Wirkstoffe – Bedaquilin und Delamanid" (in de). Österreichische Apothekerzeitung (4/2013): 22. 18 February 2013.
- ↑ "Delamanid for multidrug-resistant pulmonary tuberculosis". The New England Journal of Medicine 366 (23): 2151–2160. June 2012. doi:10.1056/NEJMoa1112433. PMID 22670901.
- ↑ "European Medicines Agency recommends two new treatment options for tuberculosis". European Medicines Agency. 22 November 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/11/news_detail_001972.jsp&mid=WC0b01ac058004d5c1.
 |

