Chemistry:Dibutylamine
From HandWiki
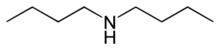
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-Butylbutan-1-amine | |
| Other names
(Dibutyl)amine
Dibutylamine (deprecated) | |
| Identifiers | |
3D model (JSmol)
|
|
| 506001 | |
| ChemSpider | |
| EC Number |
|
| MeSH | dibutylamine |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2248 |
| |
| |
| Properties | |
| C8H19N | |
| Molar mass | 129.247 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| Density | 767 mg mL−1 |
| Melting point | −61.90 °C; −79.42 °F; 211.25 K |
| Boiling point | 137 to 177 °C; 278 to 350 °F; 410 to 450 K |
| 4.7 g L−1 | |
| Vapor pressure | 340 Pa |
Henry's law
constant (kH) |
110 mol Pa−1 kg−1 |
| -103.7·10−6 cm3/mol | |
Refractive index (nD)
|
1.417 |
| Thermochemistry | |
Heat capacity (C)
|
292.9 J−1 K mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−214.8–−209.8 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−5.6534–−5.6490 MJ mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | WARNING |
| H226, H302, H312, H332 | |
| P280 | |
| Flash point | 40 °C (104 °F; 313 K) |
| 312 °C (594 °F; 585 K) | |
| Explosive limits | 1.1–10% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
360 mg kg−1 (oral, rat) |
| Related compounds | |
Related amines
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dibutylamine is an amine used as a corrosion inhibitor, in the manufacture of emulsifiers, and as a flotation agent. It is flammable and toxic.[2]
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3-160, 5-54, 8-53, 8-112, 15-18, 16-27, ISBN 0-8493-0594-2
- ↑ Gangolli, S. (1999). The Dictionary of Substances and Their Effects. London: Royal Society of Chemistry. pp. 204. ISBN 9780854048137. https://books.google.com/books?id=s4YittJrOsAC. Retrieved 2009-12-03.
 |

