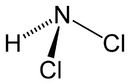
Chemistry:Dichloramine
From HandWiki
| |||
| Names | |||
|---|---|---|---|
| Other names
Chlorimide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NHCl 2 | |||
| Molar mass | 85.92 g·mol−1 | ||
| Appearance | yellow gas[1] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
Dichloramine is a reactive inorganic compound with the chemical formula NHCl
2. It is one of the three chloramines of ammonia, the others being monochloramine (NH
2Cl) and nitrogen trichloride (NCl
3). This yellow gas is unstable and reacts with many materials.[1] It is formed by a reaction between ammonia and chlorine or sodium hypochlorite. It is a byproduct formed during the synthesis of monochloramine and nitrogen trichloride.
Synthesis
Dichloramine can be prepared by a reaction between monochloramine and chlorine or sodium hypochlorite:[1]
- NH
2Cl + Cl
2 → NHCl
2 + HCl
Reactions
Dichloramine reacts with the hydroxide ion, which can be present in water or comes from water molecules, to yield nitroxyl and the chloride ion.[2]
References
- ↑ 1.0 1.1 1.2 Holleman-Wiberg: Lehrbuch der Anorganischen Chemie, 102. Auflage, Berlin 2007, ISBN 978-3-11-017770-1.
- ↑ White, George Clifford (1986). The handbook of chlorination (2nd ed.). New York: Van Nostrand Reinhold. pp. 169. ISBN 0-442-29285-6.
 |