Chemistry:Nitrogen trifluoride

| |

| |
| Names | |
|---|---|
| IUPAC name
Nitrogen trifluoride
| |
| Other names
Nitrogen fluoride
Trifluoramine Trifluorammonia | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 1551 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2451 |
| |
| |
| Properties | |
| NF3 | |
| Molar mass | 71.00 g/mol |
| Appearance | colorless gas |
| Odor | moldy |
| Density | 3.003 kg/m3 (1 atm, 15 °C) 1.885 g/cm3 (liquid at b.p.) |
| Melting point | −207.15 °C (−340.87 °F; 66.00 K) |
| Boiling point | −129.06 °C (−200.31 °F; 144.09 K) |
| 0.021 g/100 mL | |
| Vapor pressure | 44.0 atm[1](−38.5 °F or −39.2 °C or 234.0 K)[lower-alpha 1] |
Refractive index (nD)
|
1.0004 |
| Structure | |
| trigonal pyramidal | |
| 0.234 D | |
| Thermochemistry | |
Heat capacity (C)
|
53.26 J/(mol·K) |
Std molar
entropy (S |
260.3 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−31.4 kcal/mol[2] −109 kJ/mol[3] |
Gibbs free energy (ΔfG˚)
|
−84.4 kJ/mol |
| Hazards | |
| Safety data sheet | AirLiquide |
| H270, H280, H332 | |
| P220, P244, P260, P304+340, P315, P370+376, P403 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
2000 ppm (mouse, 4 h) 9600 ppm (dog, 1 h) 7500 ppm (monkey, 1 h) 6700 ppm (rat, 1 h) 7500 ppm (mouse, 1 h)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 10 ppm (29 mg/m3)[4] |
REL (Recommended)
|
TWA 10 ppm (29 mg/m3)[4] |
IDLH (Immediate danger)
|
1000 ppm[4] |
| Related compounds | |
Other anions
|
nitrogen trichloride nitrogen tribromide nitrogen triiodide ammonia |
Other cations
|
phosphorus trifluoride arsenic trifluoride antimony trifluoride bismuth trifluoride |
Related binary fluoro-azanes
|
tetrafluorohydrazine |
Related compounds
|
dinitrogen difluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitrogen trifluoride (NF3) is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics.[6] Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.[7][8]
Synthesis and reactivity
Nitrogen trifluoride did not exist in significant quantities on Earth prior to its synthesis by humans. It is a rare example of a binary fluoride that can be prepared directly from the elements only at very uncommon conditions, such as an electric discharge.[9] After first attempting the synthesis in 1903, Otto Ruff prepared nitrogen trifluoride by the electrolysis of a molten mixture of ammonium fluoride and hydrogen fluoride.[10] It proved to be far less reactive than the other nitrogen trihalides nitrogen trichloride, nitrogen tribromide and nitrogen triiodide, all of which are explosive. Alone among the nitrogen trihalides it has a negative enthalpy of formation. It is prepared in modern times both by direct reaction of ammonia and fluorine and by a variation of Ruff's method.[11] It is supplied in pressurized cylinders.
NF3 is slightly soluble in water without undergoing chemical reaction. It is nonbasic with a low dipole moment of 0.2340 D. By contrast, ammonia is basic and highly polar (1.47 D).[12] This difference arises from the fluorine atoms acting as electron-withdrawing groups, attracting essentially all of the lone pair electrons on the nitrogen atom.
Similar to dioxygen, NF3 is a potent yet sluggish oxidizer.[11] It oxidizes hydrogen chloride to chlorine:[citation needed]
- 2 NF3 + 6 HCl → 6 HF + N2 + 3 Cl2
However, it only attacks (explosively) organic compounds at high temperatures. Consequently it is compatible under standard conditions with several plastics, as well as steel and Monel.[11]
Above 200-300 °C, NF3 radicalizes to nitrogen difluoride and free fluorine radicals. In the presence of metals to remove the fluorine radicals, the mixture cools to give tetrafluorohydrazine:
- 2 NF3 + Cu → N2F4 + CuF2
NF3 reacts with fluorine and antimony pentafluoride to give the tetrafluoroammonium salt:[11]
- NF3 + F2 + SbF5 → NF+4SbF−6
Mixtures of NF3 and B2H6 are explosive even at cryogenic temperatures, reacting to produce nitrogen gas, boron trifluoride, and hydrofluoric acid.[13]
Applications
Etching
Nitrogen trifluoride is primarily used to remove silicon and silicon-compounds during the manufacturing of semiconductor devices such as LCD displays, some thin-film solar cells, and other microelectronics. In these applications NF3 is initially broken down within a plasma. The resulting fluorine radicals are the active agents that attack polysilicon, silicon nitride and silicon oxide. They can be used as well to remove tungsten silicide, tungsten, and certain other metals. In addition to serving as an etchant in device fabrication, NF3 is also widely used to clean PECVD chambers.
NF3 dissociates more readily within a low-pressure discharge in comparison to perfluorinated compounds (PFCs) and sulfur hexafluoride (SF6). The greater abundance of negatively-charged free radicals thus generated can yield higher silicon removal rates, and provide other process benefits such as less residual contamination and a lower net charge stress on the device being fabricated. As a somewhat more thoroughly consumed etching and cleaning agent, NF3 has also been promoted as an environmentally preferable substitute for SF6 or PFCs such as hexafluoroethane.[14]
The utilization efficiency of the chemicals applied in plasma processes varies widely between equipment and applications. A sizeable fraction of the reactants are wasted into the exhaust stream and can ultimately be emitted into Earth's atmosphere. Modern abatement systems can substantially decrease atmospheric emissions.[15] NF3 has not been subject to significant use restrictions. The annual reporting of NF3 production, consumption, and waste emissions by large manufacturers has been required in many industrialized countries as a response to the observed atmospheric growth and the international Kyoto Protocol.[16]
Highly toxic fluorine gas (F2, diatomic fluorine) is a climate neutral replacement for nitrogen trifluoride in some manufacturing applications. It requires more stringent handling and safety precautions, especially to protect manufacturing personnel.[17]
Nitrogen trifluoride is also used in hydrogen fluoride and deuterium fluoride lasers, which are types of chemical lasers. There it is also preferred to fluorine gas due to its more convenient handling properties
Greenhouse gas
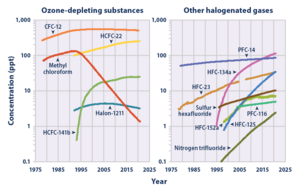
NF3 is a greenhouse gas, with a global warming potential (GWP) 17,200 times greater than that of CO2 when compared over a 100-year period.[18][19][20] Its GWP place it second only to SF6 in the group of Kyoto-recognised greenhouse gases, and NF3 was included in that grouping with effect from 2013 and the commencement of the second commitment period of the Kyoto Protocol. It has an estimated atmospheric lifetime of 740 years,[18] although other work suggests a slightly shorter lifetime of 550 years (and a corresponding GWP of 16,800).[21]
Although NF3 has a high GWP, for a long time its radiative forcing in the Earth's atmosphere has been assumed to be small, spuriously presuming that only small quantities are released into the atmosphere. Industrial applications of NF3 routinely break it down, while in the past previously used regulated compounds such as SF6 and PFCs were often released. Research has questioned the previous assumptions. High-volume applications such as DRAM computer memory production, the manufacturing of flat panel displays and the large-scale production of thin-film solar cells use NF3.[21][22]
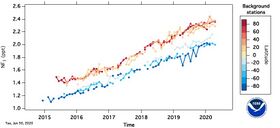
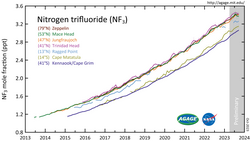
Since 1992, when less than 100 tons were produced, production has grown to an estimated 4000 tons in 2007 and is projected to increase significantly.[21] World production of NF3 is expected to reach 8000 tons a year by 2010. By far the world's largest producer of NF3 is the US industrial gas and chemical company Air Products & Chemicals. An estimated 2% of produced NF3 is released into the atmosphere.[23][24] Robson projected that the maximum atmospheric concentration is less than 0.16 parts per trillion (ppt) by volume, which will provide less than 0.001 Wm−2 of IR forcing.[25] The mean global tropospheric concentration of NF3 has risen from about 0.02 ppt (parts per trillion, dry air mole fraction) in 1980, to 0.86 ppt in 2011, with a rate of increase of 0.095 ppt yr−1, or about 11% per year, and an interhemispheric gradient that is consistent with emissions occurring overwhelmingly in the Northern Hemisphere, as expected. This rise rate in 2011 corresponds to about 1200 metric tons/y NF3 emissions globally, or about 10% of the NF3 global production estimates. This is a significantly higher percentage than has been estimated by industry, and thus strengthens the case for inventorying NF3 production and for regulating its emissions.[26] One study co-authored by industry representatives suggests that the contribution of the NF3 emissions to the overall greenhouse gas budget of thin-film Si-solar cell manufacturing is clear.[27]
The UNFCCC, within the context of the Kyoto Protocol, decided to include nitrogen trifluoride in the second Kyoto Protocol compliance period, which begins in 2012 and ends in either 2017 or 2020. Following suit, the WBCSD/WRI GHG Protocol is amending all of its standards (corporate, product and Scope 3) to also cover NF3.[28]
Safety
Skin contact with NF3 is not hazardous, and it is a relatively minor irritant to mucous membranes and eyes. It is a pulmonary irritant with a toxicity considerably lower than nitrogen oxides, and overexposure via inhalation causes the conversion of hemoglobin in blood to methemoglobin, which can lead to the condition methemoglobinemia.[29] The National Institute for Occupational Safety and Health (NIOSH) specifies that the concentration that is immediately dangerous to life or health (IDLH value) is 1,000 ppm.[30]
See also
Notes
- ↑ This vapour pressure is the pressure at its critical temperature – below ordinary room temperature.
References
- ↑ Air Products; Physical Properties for Nitrogen Trifluoride
- ↑ Sinke, G. C. (1967). "The enthalpy of dissociation of nitrogen trifluoride". J. Phys. Chem. 71 (2): 359–360. doi:10.1021/j100861a022.
- ↑ Inorganic Chemistry, p. 462, at Google Books
- ↑ 4.0 4.1 4.2 NIOSH Pocket Guide to Chemical Hazards. "#0455". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0455.html.
- ↑ "Nitrogen trifluoride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7783542.html.
- ↑ Richard Conniff (2008-11-13). "The Greenhouse Gas That Nobody Knew". Yale School of Environment. https://e360.yale.edu/features/the_greenhouse_gasthat_nobody_knew.
- ↑ 7.0 7.1 "Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases - Figure 4". U.S. Environmental Protection Agency. 27 June 2016. https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases.
- ↑ 8.0 8.1 "Atmospheric Flask NF3". National Oceanic and Atmospheric Administration. 2020-06-30. https://www.esrl.noaa.gov/gmd/hats/gases/NF3.html.
- ↑ Lidin, P. A.; Molochko, V. A.; Andreeva, L. L. (1995) (in ru). pp. 442–455. ISBN 978-1-56700-041-2.
- ↑ Otto Ruff, Joseph Fischer, Fritz Luft (1928). "Das Stickstoff-3-fluorid". Zeitschrift für Anorganische und Allgemeine Chemie 172 (1): 417–425. doi:10.1002/zaac.19281720132.
- ↑ 11.0 11.1 11.2 11.3 Philip B. Henderson, Andrew J. Woytek "Fluorine Compounds, Inorganic, Nitrogen" in Kirk‑Othmer Encyclopedia of Chemical Technology, 1994, John Wiley & Sons, NY. doi:10.1002/0471238961.1409201808051404.a01 Article Online Posting Date: December 4, 2000
- ↑ Klapötke, Thomas M. (2006). "Nitrogen–fluorine compounds". Journal of Fluorine Chemistry 127 (6): 679–687. doi:10.1016/j.jfluchem.2006.03.001.
- ↑ Parry, Robert W., and Thomas C. Bissot. "The Preparation and Properties of Phosphorus Trifluoride-Borane and Phosphorus Trifluoride-Borane-d31." Journal of the American Chemical Society 78, no. 8 (1956): 1524-1527.
- ↑ H. Reichardt , A. Frenzel and K. Schober (2001). "Environmentally friendly wafer production: NF3 remote microwave plasma for chamber cleaning". Microelectronic Engineering 56 (1–2): 73–76. doi:10.1016/S0167-9317(00)00505-0.
- ↑ "F-GHG Emissions Reduction Efforts: Flat Panel Display Supplier Profiles". U.S. EPA. 2016-09-30. https://www.epa.gov/sites/production/files/2016-09/documents/fpd_full_supplier_profiles_2016_508compliant.pdf.
- ↑ "Fluorinated Greenhouse Gas Emissions and Supplies Reported to the Greenhouse Gas Reporting Program (GHGRP)". U.S. Environmental Protection Agency. 27 September 2015. https://www.epa.gov/ghgreporting/fluorinated-greenhouse-gas-emissions-and-supplies-reported-ghgrp.
- ↑ J. Oshinowo; A. Riva; M Pittroff; T. Schwarze; R. Wieland (2009). "Etch performance of Ar/N2/F2 for CVD/ALD chamber clean". Solid State Technology 52 (2): 20–24.
- ↑ 18.0 18.1 Climate Change 2007: The Physical Sciences Basis. IPCC. http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter2.pdf. Retrieved 2008-07-03.
- ↑ Robson, J. I.; Gohar, L. K.; Hurley, M. D.; Shine, K. P.; Wallington, T. (2006). "Revised IR spectrum, radiative efficiency and global warming potential of nitrogen trifluoride". Geophys. Res. Lett. 33 (10): L10817. doi:10.1029/2006GL026210. Bibcode: 2006GeoRL..3310817R.
- ↑ Richard Morgan (2008-09-01). "Beyond Carbon: Scientists Worry About Nitrogen's Effects". The New York Times. https://www.nytimes.com/2008/09/02/science/02nitr.html?ref=science.
- ↑ 21.0 21.1 21.2 Prather, M.J.; Hsu, J. (2008). "NF3, the greenhouse gas missing from Kyoto". Geophys. Res. Lett. 35 (12): L12810. doi:10.1029/2008GL034542. Bibcode: 2008GeoRL..3512810P. http://www.agu.org/journals/gl/gl0812/2008GL034542/.
- ↑ Tsai, W.-T. (2008). "Environmental and health risk analysis of nitrogen trifluoride (NF3), a toxic and potent greenhouse gas". J. Hazard. Mater. 159 (2–3): 257–63. doi:10.1016/j.jhazmat.2008.02.023. PMID 18378075.
- ↑ M. Roosevelt (2008-07-08). "A climate threat from flat TVs, microchips". Los Angeles Times. http://www.latimes.com/news/nationworld/nation/la-na-climate8-2008jul08,0,7460950.story.
- ↑ Hoag, Hannah (2008-07-10). "The Missing Greenhouse Gas". Nature Reports Climate Change (Nature News) 1 (808): pp. 99–100. doi:10.1038/climate.2008.72. http://www.nature.com/climate/2008/0808/full/climate.2008.72.html.
- ↑ Robson, Jon. Nitrogen trifluoride (NF3). Royal Meteorological Society. http://www.rmets.org/activities/awards/scholarships/sch_2.php. Retrieved 2008-10-27.
- ↑ Arnold, Tim; Harth, C. M.; Mühle, J.; Manning, A. J.; Salameh, P. K.; Kim, J.; Ivy, D. J.; Steele, L. P. et al. (2013-02-05). "Nitrogen trifluoride global emissions estimated from updated atmospheric measurements". Proc. Natl. Acad. Sci. USA 110 (6): 2029–2034. doi:10.1073/pnas.1212346110. PMID 23341630. Bibcode: 2013PNAS..110.2029A.
- ↑ V. Fthenakis; D. O. Clark; M. Moalem; M. P. Chandler; R. G. Ridgeway; F. E. Hulbert; D. B. Cooper; P. J. Maroulis (2010-10-25). "Life-Cycle Nitrogen Trifluoride Emissions from Photovoltaics". Environ. Sci. Technol. (American Chemical Society) 44 (22): 8750–7. doi:10.1021/es100401y. PMID 21067246. Bibcode: 2010EnST...44.8750F.
- ↑ Rivers, Ali (2012-08-15). "Nitrogen trifluoride: the new mandatory Kyoto Protocol greenhouse gas". Ecometrica.com (www.ecometrica.com). http://ecometrica.com/blog/nitrogen-trifluoride-the-7th-mandatory-kyoto-protocol-greenhouse-gas.
- ↑ Malik, Yogender (2008-07-03). "Nitrogen trifluoride – Cleaning up in electronic applications". Gasworld. http://www.gasworld.com/news.php?a=2896.
- ↑ "Immediately Dangerous to Life or Health Concentrations (IDLH): Nitrogen Trifluoride". National Institute for Occupational Safety and Health. 2 November 2018. https://www.cdc.gov/niosh/idlh/7783542.html.
External links
- NF3 Code of Practice (European Industrial Gas Association)]
- WebBook page for NF3
- CDC - NIOSH Pocket Guide to Chemical Hazards
| NH3 | He(N2)11 | ||||||||||||||||
| Li3N | Be3N2 | BN | β-C3N4 g-C3N4 |
N2 | NxOy | NF3 | Ne | ||||||||||
| Na3N | Mg3N2 | AlN | Si3N4 | PN P3N5 |
SxNy SN S4N4 |
NCl3 | Ar | ||||||||||
| K3N | Ca3N2 | ScN | TiN | VN | CrN Cr2N |
MnxNy | FexNy | CoN | Ni3N | CuN | Zn3N2 | GaN | Ge3N4 | As | Se | NBr3 | Kr |
| Rb3N | Sr3N2 | YN | ZrN | NbN | β-Mo2N | Tc | Ru | Rh | PdN | Ag3N | CdN | InN | Sn | Sb | Te | NI3 | Xe |
| Cs3N | Ba3N2 | Hf3N4 | TaN | WN | Re | Os | Ir | Pt | Au | Hg3N2 | TlN | Pb | BiN | Po | At | Rn | |
| Fr3N | Ra3N | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | CeN | Pr | Nd | Pm | Sm | Eu | GdN | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UN | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |

